Addressing Acetaminophen Interference in Implantable Biosensors: Mechanisms, Mitigation Strategies, and Clinical Implications
This article provides a comprehensive analysis of acetaminophen interference in implantable biosensors, a critical challenge for researchers and drug development professionals in the field of continuous monitoring.
Addressing Acetaminophen Interference in Implantable Biosensors: Mechanisms, Mitigation Strategies, and Clinical Implications
Abstract
This article provides a comprehensive analysis of acetaminophen interference in implantable biosensors, a critical challenge for researchers and drug development professionals in the field of continuous monitoring. It explores the foundational electrochemical mechanisms underlying this interference, particularly in first-generation glucose oxidase-based sensors. The content reviews advanced methodological approaches for interference suppression, including membrane technologies and electrochemical techniques. Furthermore, it evaluates troubleshooting protocols, sensor optimization strategies, and comparative performance data across commercial biosensor platforms. This synthesis of current research and development offers valuable insights for creating more robust and reliable implantable diagnostic devices, ultimately enhancing patient safety in clinical applications involving polypharmacy.
The Acetaminophen Interference Problem: Foundational Science and Clinical Impact
Frequently Asked Questions (FAQs)
1. What is electrochemical interference and why is it a problem for implantable biosensors? Electrochemical interference occurs when electroactive species other than the target analyte produce a false signal in a biosensor. In implantable glucose sensors, this is a significant problem because substances like acetaminophen and ascorbic acid are readily oxidized at the same potential used to detect hydrogen peroxide (the product of the glucose oxidase reaction). This leads to an overestimation of glucose concentration, which can be dangerous for patients, particularly those using the sensor for diabetes management [1] [2].
2. Why is acetaminophen a particularly serious interferent? Acetaminophen is a widely used over-the-counter pain and fever medication. It is electroactive and gets oxidized at the working electrode of first-generation amperometric biosensors, which typically operate at a high potential (e.g., +0.6 V vs. Ag/AgCl). Studies have shown that a clinically common plasma acetaminophen concentration of 200 μmol/L can cause a significant positive bias, leading to an underestimation of glucose concentration by approximately 6-7 mmol/L, which is a critical error [3] [2].
3. What are the main strategies to eliminate or reduce acetaminophen interference? Researchers have developed several key strategies to mitigate this interference:
- Permselective Membranes: Using composite membranes, such as cellulose acetate combined with Nafion, to create a charged barrier that selectively excludes interferents like acetaminophen while allowing hydrogen peroxide to pass [3] [1].
- Lowering Operating Potential: Employing second-generation biosensors that use artificial electron mediators, allowing the sensor to operate at a much lower potential where acetaminophen is not oxidized [4] [5].
- Electrode Design: Incorporating specific membrane "domains," such as interference membranes and bioprotective membranes, designed to reduce the flux of interfering substances to the electrode surface [4].
4. How do I test for interference in my sensor experiment? A standard in vitro protocol involves measuring the sensor's response to a target glucose concentration (e.g., 5 mmol/L) and then measuring the response after adding a physiological concentration of the interferent (e.g., 100-200 μmol/L acetaminophen). The bias is calculated as the difference in signal. For example, one study found that 100 μmol/L ascorbate introduced a minimal bias of ~0.4 mmol/L glucose, whereas 200 μmol/L acetaminophen introduced a ~7 mmol/L bias [2].
5. Are commercial Continuous Glucose Monitors (CGMs) affected by acetaminophen? Yes, many first-generation electrochemical biosensor-based CGMs are affected. Manufacturer labels for devices like the Dexcom G6/G7 and Medtronic Guardian Connect explicitly warn that taking higher-than-maximum dosages of acetaminophen may falsely raise sensor glucose readings. Some newer sensor models have incorporated design improvements, such as permselective membranes, to reduce this effect [4].
Troubleshooting Guide
Problem: High Background Signal or Inaccurate Readings in Complex Media
Potential Cause: Interference from electroactive species like acetaminophen, ascorbic acid, or uric acid.
Solution:
- Apply a Permselective Membrane: Spin-coat or dip-coat the sensor with a composite membrane. A proven methodology is:
- Prepare a solution of Cellulose Acetate (e.g., 2-5% w/v in acetone) and Nafion (e.g., 0.5-2% w/v in lower aliphatic alcohols/water).
- Apply this solution over the sensor's enzyme layer and allow it to dry, forming a thin film.
- Mechanism: Cellulose acetate acts as a size-exclusion layer, while Nafion, being negatively charged, repels anionic interferents like ascorbate and urate. For neutral molecules like acetaminophen, the composite structure creates a diffusion barrier, significantly slowing its response time. When combined with the drug's rapid clearance in the body, this minimizes its impact in vivo [3].
- Switch to a Mediated (Second-Generation) Biosensor Design:
- Immobilize both Glucose Oxidase and a mediator (e.g., ferrocene derivatives, ferricyanide) within a polymer matrix on the electrode.
- Mechanism: The mediator shuttles electrons from the reduced enzyme to the electrode, allowing the operating potential to be lowered to a range (e.g., 0.0 V to +0.2 V) where most common interferents are not electroactive [4] [5].
Problem: Sensor Signal Drift or Loss of Sensitivity Post-Implantation
Potential Cause: Biofouling, where proteins and cells adhere to the sensor surface, causing a foreign body response and limiting analyte diffusion.
Solution:
- Incorporate a Bioprotective Membrane: Use a outermost membrane designed for biocompatibility. Materials like poly(ethylene glycol) (PEG)-based hydrogels or polyurethane derivatives can reduce protein adsorption and cell adhesion, extending the functional life of the sensor in vivo [6] [4].
- Utilize Smart Biomaterials: Investigate the use of biodegradable and self-healing materials. These advanced materials can improve biocompatibility and device longevity, reducing the host's immune response over time [6] [7].
Quantitative Data on Interference
The table below summarizes the typical interference impact of key substances on first-generation amperometric glucose biosensors.
Table 1: Quantifying Interference in Glucose Biosensors
| Interfering Substance | Physiological Concentration Range | Approximate Signal Bias (vs. Glucose) | Key Mitigation Strategy |
|---|---|---|---|
| Acetaminophen | Up to 200 μmol/L (therapeutic) | ~7 mmol/L glucose error [2] | Composite membranes (Cellulose Acetate/Nafion) [3] |
| Ascorbic Acid (Vitamin C) | 50-100 μmol/L | ~0.4 mmol/L glucose error (at 100 μmol/L) [2] | Nafion membrane, Ascorbate Oxidase [1] [5] |
| Uric Acid | 200-500 μmol/L | Varies | Nafion membrane [1] |
Experimental Protocols
Protocol 1: Evaluating Interference with a Permselective Membrane
Objective: To test the effectiveness of a cellulose acetate/Nafion composite membrane against acetaminophen interference.
Materials:
- Fabricated glucose biosensor (Pt working electrode with immobilized Glucose Oxidase)
- Cellulose acetate (CA)
- Nafion perfluorinated resin solution
- Acetone
- Phosphate Buffered Saline (PBS), pH 7.4
- Glucose stock solution
- Acetaminophen stock solution
- Electrochemical workstation (e.g., potentiostat)
Procedure:
- Membrane Preparation: Prepare a 3% (w/v) solution of CA in acetone. Mix this 1:1 by volume with a 1% (w/v) Nafion solution.
- Sensor Modification: Dip-coat the fabricated glucose sensor into the CA/Nafion solution and withdraw it slowly. Allow the sensor to dry at room temperature for 1 hour.
- Amperometric Measurement:
- Set the potentiostat to apply a constant potential of +0.6 V vs. Ag/AgCl.
- Immerse the sensor in stirred PBS and record the baseline current.
- Add glucose to achieve a 5 mM concentration and record the steady-state current (Iglucose).
- Rinse the sensor and re-establish baseline in fresh PBS.
- Add acetaminophen to achieve a 200 μM concentration and record the steady-state current (Iacetaminophen).
- Data Analysis: Calculate the degree of interference by comparing I_acetaminophen to the current expected from an equimolar glucose solution. A well-functioning membrane will show a drastically reduced response to acetaminophen [3] [1].
Protocol 2: Testing a Mediated Biosensor at Low Potential
Objective: To demonstrate reduced acetaminophen interference by operating a biosensor at a low working potential.
Materials:
- Fabricated mediated biosensor (e.g., with Ferrocene carboxylic acid / Glucose Oxidase in a polymer matrix)
- PBS, pH 7.4
- Glucose stock solution
- Acetaminophen stock solution
- Electrochemical workstation
Procedure:
- Amperometric Measurement at Low Potential:
- Set the potentiostat to apply a constant potential of +0.2 V vs. Ag/AgCl.
- Repeat Step 3 from Protocol 1, measuring the sensor's response to 5 mM glucose and then to 200 μM acetaminophen.
- Data Analysis: The current response from acetaminophen at this low potential should be negligible compared to the response from glucose, confirming the success of the mediated approach in eliminating this interference [4] [5].
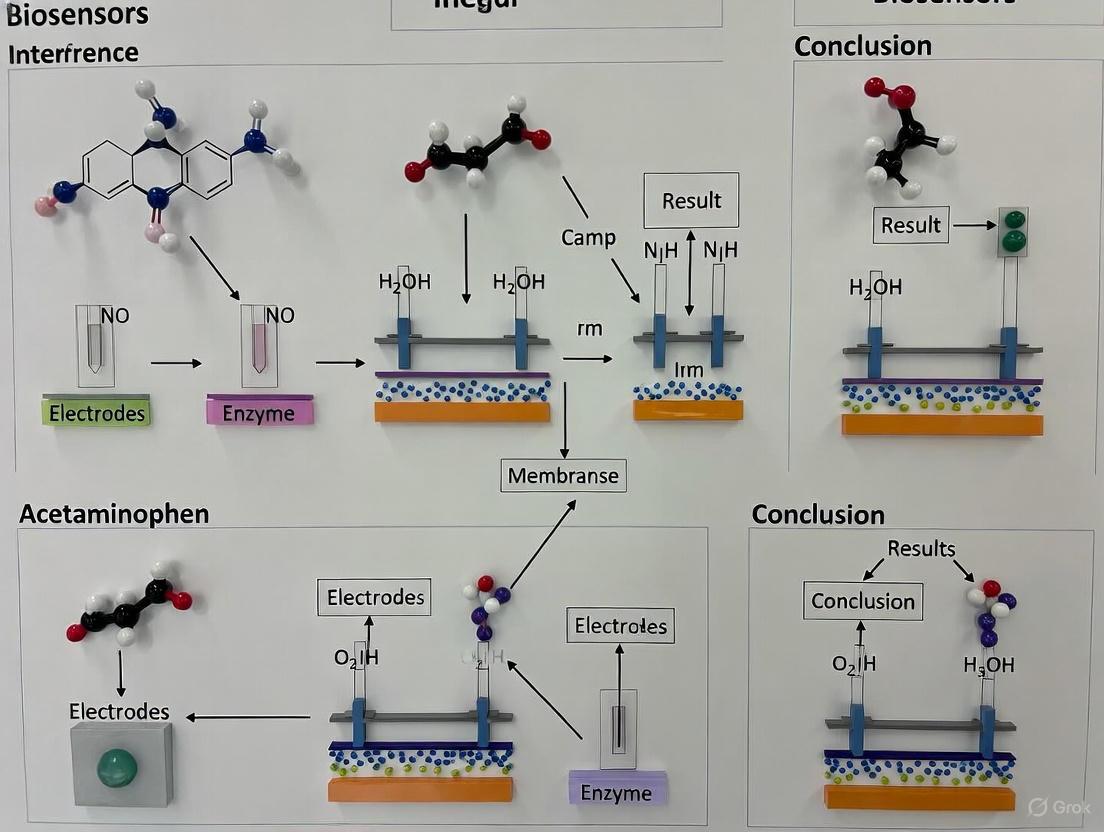
Visual Experimental Workflows
Interference Mechanism and Mitigation Pathways
In-Vitro Interference Testing Workflow
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Mitigating Acetaminophen Interference
| Reagent/Material | Function/Benefit | Key Consideration |
|---|---|---|
| Nafion | A cationic perfluorosulfonated polymer. Creates a negatively charged membrane that repels anionic interferents like ascorbate and urate [3] [5]. | Less effective against neutral interferents like acetaminophen alone; often used in composites. |
| Cellulose Acetate | A polymer that forms a size-selective hydrogel membrane. Restricts the diffusion of larger molecules towards the electrode surface [3] [1]. | The ratio with other polymers (e.g., Nafion) is critical for optimizing selectivity and Hâ‚‚Oâ‚‚ diffusion. |
| Ferrocene and Derivatives | Common redox mediators. Shuttle electrons from Glucose Oxidase to the electrode, enabling low-potential operation and minimizing interferent oxidation [4] [5]. | Must be immobilized effectively to prevent leakage from the sensor over time. |
| Polyurethane / PEG Hydrogels | Used as outer bioprotective membranes. Improve biocompatibility, reduce biofouling, and can also contribute to controlling analyte and interferent diffusion [6] [4]. | Mechanical properties and porosity must be tuned to match the implantation site. |
| Nicarbazin-d8 | Nicarbazin-d8, MF:C19H18N6O6, MW:434.4 g/mol | Chemical Reagent |
| ATM Inhibitor-2 | ATM Inhibitor-2|ATM Kinase Inhibitor|Research Compound | ATM Inhibitor-2 is a potent, selective ATM kinase inhibitor used in cancer research and DNA damage response (DDR) studies. For Research Use Only. Not for human use. |
Technical FAQ: Understanding and Troubleshooting Acetaminophen Interference
Q1: What is the fundamental mechanism by which acetaminophen interferes with CGM readings?
Acetaminophen interferes with the electrochemical sensing principle used by many continuous glucose monitors (CGMs), specifically those of a first-generation biosensor design [4]. These systems, including certain models from Dexcom and Medtronic, use the enzyme glucose oxidase (GOx) to detect glucose [4].
The interference occurs because the sensor does not perfectly distinguish the signal generated by glucose from that generated by other easily oxidized substances [8] [9]. When glucose in the interstitial fluid passes through the GOx membrane, it produces hydrogen peroxide (Hâ‚‚Oâ‚‚). The sensor applies a voltage, causing the Hâ‚‚Oâ‚‚ to decompose and release electrons, which are then converted into a glucose reading [8] [9]. Acetaminophen is also easily oxidized at a similar voltage, causing it to generate an additional, non-glucose-related electrical current. The CGM system mistakenly interprets this combined signal as an elevated glucose level, resulting in a falsely high reading [8] [9].
Q2: How does the interference from intravenous (IV) acetaminophen differ from oral administration?
The route of administration significantly impacts the severity of interference. Intravenous acetaminophen leads to a higher and more rapid peak in blood concentration compared to oral administration, which in turn causes a greater and more acute overestimation of glucose levels by the CGM [8] [9].
The following table summarizes the key quantitative findings from a clinical case study on IV acetaminophen interference with a Medtronic Guardian 4 sensor [8] [9]:
| Parameter | Findings from IV Acetaminophen Case Study |
|---|---|
| Dosage | 15 mg/kg administered intravenously over 15 minutes [8] [9] |
| Time to Peak CGM Error | 29.2 ± 1.9 minutes (mean ± standard deviation) after administration [8] [9] |
| Magnitude of CGM Error | Estimated discrepancy of 55 to 114 mg/dL compared to capillary blood glucose measurements [8] [9] |
| Relationship to Glucose Level | Significant negative correlation; discrepancies were greater at lower blood glucose levels [8] [9] |
| Duration of Interference | Discrepancies persisted for more than 2 hours [8] [9] |
Q3: What specific risk does this interference pose in automated insulin delivery (AID) systems?
Falsely elevated CGM readings pose a critical safety risk in AID systems, also known as closed-loop systems [8] [9]. These systems rely on real-time CGM data to make automated decisions on insulin dosing. A falsely high glucose reading could trigger the system to deliver an unneeded "autocorrection" insulin bolus [8] [9]. This inappropriate insulin delivery, occurring when the patient's actual blood glucose is normal or low, significantly increases the risk of iatrogenic hypoglycemia [8] [9].
Q4: What is the recommended clinical troubleshooting protocol for patients requiring IV acetaminophen?
When a patient using a CGM requires IV acetaminophen, clinicians should adopt the following protocol to mitigate risk [8] [9]:
- Awareness and Recognition: Be aware that IV acetaminophen causes significant CGM interference, with errors potentially exceeding 100 mg/dL.
- Verify with Blood Glucose Meter: Do not rely on CGM readings during and for at least 2-3 hours after IV acetaminophen administration. Instead, use a blood glucose meter to guide therapy [8] [9].
- Suspend Automated Insulin Delivery: For patients on AID systems, switch the insulin pump to manual mode before administering IV acetaminophen to prevent automated correction boluses based on erroneous data [8] [9].
- Resume AID with Caution: Only switch the pump back to automated mode after confirming via blood glucose meter that the interference has subsided and the CGM readings have realigned with actual blood glucose levels [8] [9].
Experimental Insights for Research & Development
Experimental Protocol: Quantifying Acetaminophen Interference
The following methodology, adapted from a published case report, provides a framework for systematically evaluating sensor interference in a clinical or research setting [8] [9].
Objective: To quantify the magnitude, timing, and duration of CGM error induced by intravenous acetaminophen.
Materials & Reagents:
- CGM System: The sensor system under investigation (e.g., Medtronic Guardian 4) [8] [9].
- Blood Glucose Monitor (BGM): A validated, FDA-cleared device for reference capillary blood glucose measurements (e.g., ACCU-CHEK Guide Link) [8] [9].
- Intravenous Acetaminophen: Prepared at the standard dosage (e.g., 15 mg/kg) [8] [9].
- Data Extraction Software: Tool for extracting timestamped CGM data from the proprietary system (e.g., from the insulin pump) [8] [9].
- Statistical Software: For data analysis and linear regression (e.g., R software) [8] [9].
Procedure:
- Baseline Period: Ensure no oral intake or significant insulin boluses for at least 2 hours prior to acetaminophen administration to stabilize glucose levels [8] [9].
- Administration: Administer the IV acetaminophen dose over the specified duration (e.g., 15 minutes) [8] [9].
- Data Collection:
- Data Analysis:
- Peak Identification: Use CGM data to identify the peak glucose reading and the time to peak after administration [8] [9].
- Error Calculation: Calculate the discrepancy (CGM reading - BGM reading) at each time point.
- Linear Regression: Perform regression analysis to assess the relationship between the reference blood glucose level and the magnitude of the CGM discrepancy [8] [9].
Research Reagent Solutions & Materials
The table below lists key materials and reagents relevant to researching interference in implantable biosensors.
| Item | Function/Application in Research |
|---|---|
| Glucose Oxidase (GOx) | The primary enzyme used in first-generation electrochemical biosensors for glucose recognition; central to the interference mechanism [4]. |
| Acetaminophen (IV Formulation) | The interfering substance used to challenge sensor performance and quantify susceptibility in experimental protocols [8] [9]. |
| Platinum (Pt) Electrode | A common material for the working electrode in first-generation CGM designs where the oxidation reaction occurs [4] [10]. |
| Permselective Membrane | A sensor design feature (e.g., in Dexcom G6/G7) intended to reduce the flux of interfering substances like acetaminophen to the electrode surface [4]. |
| Hydrogen Peroxide (Hâ‚‚Oâ‚‚) | The product of the glucose oxidase reaction; its electrochemical detection is the source of the signal that acetaminophen disrupts [4] [8]. |
| Biocompatible Polymers (e.g., Parylene-C) | Used for device insulation and encapsulation to improve biocompatibility and reduce the foreign body response in implantable sensors [10]. |
Biosensor Interference Pathway
The following diagram illustrates the core electrochemical mechanism by which acetaminophen causes interference in first-generation biosensors.
Acetaminophen (paracetamol) is one of the most well-documented and serious electrochemical interferences for oxidase-based amperometric biosensors. For researchers developing implantable glucose sensors, understanding and mitigating this interference is crucial for ensuring accurate physiological measurements. This technical guide examines the underlying mechanisms of acetaminophen interference, provides experimental methodologies for its investigation, and summarizes current strategies to eliminate its effects, providing a foundation for robust biosensor design.
The Electrochemical Mechanism of Interference
Core Sensing Principle of GOx-Based Sensors
Most continuous glucose monitors (CGMs) and implantable glucose sensors are amperometric biosensors that rely on glucose oxidase (GOx) as their molecular recognition element. The canonical reaction sequence is as follows [11] [12]:
- Enzymatic Reaction: Glucose oxidase catalyzes the oxidation of β-D-glucose to D-glucono-1,5-lactone, while simultaneously reducing the enzyme's cofactor, flavin adenine dinucleotide (FAD), to FADH₂.
Glucose + GOx(FAD) → Gluconolactone + GOx(FADH₂) - Enzyme Re-oxidation: The reduced enzyme is re-oxidized by molecular oxygen (O₂), producing hydrogen peroxide (H₂O₂).
GOx(FADH₂) + O₂ → GOx(FAD) + H₂O₂ - Electrochemical Detection: In first-generation sensors, H₂O₂ is oxidized at a positively polarized working electrode (typically +0.6 V to +0.7 V vs. Ag/AgCl), generating an electrical current proportional to the glucose concentration.
Hâ‚‚Oâ‚‚ → Oâ‚‚ + 2H⺠+ 2eâ»
Competitive Oxidation of Acetaminophen
The interference occurs at the final detection step. At the high working potential required for H₂O₂ oxidation, acetaminophen—which contains a readily oxidizable phenolic hydroxyl group—is also oxidized at the electrode surface [13] [11]. The electrochemical oxidation of acetaminophen produces N-acetyl-p-benzoquinone imine (NAPQI) and releases electrons [14] [11]:
Acetaminophen → NAPQI + 2H⺠+ 2eâ»
The critical issue is that the sensor's electronics cannot distinguish between the electrons generated from the target analyte (Hâ‚‚Oâ‚‚, derived from glucose) and those from the interfering species (acetaminophen). Consequently, the oxidation current is additive, leading to a falsely elevated glucose reading [13] [14] [8].
The diagram below illustrates this competitive interference mechanism at the sensor electrode.
Quantitative Analysis of Interference Effects
The magnitude of acetaminophen interference is dose-dependent and can be significant, especially at lower glucose concentrations. The following table summarizes key quantitative findings from clinical and experimental studies.
Table 1: Quantified Impact of Acetaminophen on Glucose Sensor Readings
| Acetaminophen Dose & Route | Sensor Model(s) Tested | Observed Discrepancy (CGM vs. Reference) | Time to Peak Interference | Citation |
|---|---|---|---|---|
| 1,000 mg (Oral) | Dexcom G4 | Mean difference: 61 mg/dL (Upper 95% CI: 77 mg/dL) | 120 minutes | [13] |
| 1,000 mg (Oral) | Dexcom Seven Plus, Medtronic Guardian, Dexcom G4 Platinum | CGM readings: ~85 to 400 mg/dL (Reference BG: ~90 mg/dL) | Coincided with peak ISF acetaminophen | [14] |
| 15 mg/kg (IV) | Medtronic Guardian 4 | Estimated discrepancy: 55 to 114 mg/dL | 29.2 ± 1.9 minutes | [8] |
| 1,000 mg (Oral) | Guardian REAL-TIME | Increase from baseline: 21 mg/dL (at BG ~90 mg/dL) | Not Specified | [8] |
| 1,000 mg (Oral) | Dexcom G4 Platinum | Increase from baseline: 30 mg/dL (at BG ~90 mg/dL) | Not Specified | [8] |
A critical finding for patient safety is that the interference effect is inversely correlated with blood glucose levels. Analysis of IV acetaminophen administration showed a significant negative correlation, where the discrepancy between CGM readings and actual blood glucose was greater at lower glucose levels, thereby increasing the risk of masked hypoglycemia [8].
Experimental Protocols for Investigating Interference
Researchers can use the following methodologies to characterize and quantify acetaminophen interference in sensor systems.
In-Vitro Interference Challenge Protocol
This protocol is suitable for initial screening of sensor materials or designs.
- Objective: To quantify the amperometric response of a GOx sensor to acetaminophen in a controlled buffer system.
- Materials:
- Potentiostat and electrochemical cell.
- Working electrode (fabricated sensor), counter electrode, and reference electrode (Ag/AgCl).
- Phosphate Buffered Saline (PBS), pH 7.4.
- Stock solutions of D-glucose and acetaminophen in PBS.
- Procedure:
- Place the sensor in PBS under stirring conditions and apply the standard working potential (e.g., +0.65 V).
- Allow the background current to stabilize.
- Successively add aliquots of glucose stock solution to achieve desired concentrations (e.g., 50, 100, 200 mg/dL). Record the steady-state current after each addition.
- Rinse the sensor and cell. Re-stabilize the baseline current in fresh PBS.
- Successively add aliquots of acetaminophen stock solution across a physiological range (e.g., 0, 5, 10, 20 mg/L). Record the steady-state current after each addition.
- Data Analysis: Calculate the apparent "glucose-equivalent" signal generated by acetaminophen by comparing the current density per mg/dL of glucose to the current density per mg/L of acetaminophen.
In-Vivo Microdialysis and Sensor Correlation Protocol
This advanced method provides direct evidence of interference in a physiological context by simultaneously measuring interstitial fluid (ISF) drug concentrations and sensor performance [14].
- Objective: To correlate interstitial acetaminophen pharmacokinetics with the temporal profile of CGM sensor error.
- Materials:
- CGM systems (e.g., Dexcom, Medtronic Guardian).
- Microdialysis system with abdominal subcutaneous catheters.
- YSI analyzer or equivalent for reference plasma glucose.
- HPLC or COBAS c311 analyzer for plasma and microdialysate acetaminophen concentration.
- Procedure:
- In healthy volunteers or animal models, insert CGM sensors and microdialysis catheters in close proximity in abdominal subcutaneous tissue.
- After equilibration, administer a standard dose of acetaminophen (e.g., 1 g orally or 15 mg/kg IV).
- Collect serial blood and microdialysate samples at baseline and periodic intervals post-administration.
- Analyze samples for glucose and acetaminophen concentrations.
- Continuously record CGM glucose values.
- Data Analysis:
- Plot plasma glucose, ISF acetaminophen, and CGM glucose over time.
- The interference is directly demonstrated when CGM readings spike while plasma glucose remains constant, and this spike temporally aligns with the rise of acetaminophen in the ISF [14].
Mitigation Strategies: From Membranes to New Materials
Several strategies have been developed to minimize or eliminate acetaminophen interference, primarily focused on creating a selective barrier.
Permselective Membranes
The most established approach involves coating the sensor with a polymer membrane that selectively allows Hâ‚‚Oâ‚‚ to pass while blocking larger or differently charged molecules like acetaminophen.
- Cellulose Acetate and Nafion Composite: A classic and effective solution is a composite membrane of cellulose acetate and Nafion. The cellulose acetate layer acts as a size-exclusion barrier, while the charged Nafion layer can repel acetaminophen based on its ionic properties. This combination was shown to effectively eliminate acetaminophen interference in an implantable sensor while maintaining reasonable Hâ‚‚Oâ‚‚ diffusivity [3] [12].
- Electropolymerized Films: Membranes like polyphenylenediamine (PPD) can be electrochemically synthesized directly on the electrode surface. These films form dense, non-conducting layers with pore sizes that are tunable to exclude interferents like ascorbic acid and acetaminophen [12].
Advanced Material Strategies
Recent research explores novel materials and concepts to push the boundaries of interference rejection.
- Conductive Membrane Encapsulation: A novel strategy involves encapsulating the sensor with a conductive membrane (e.g., gold-coated track-etch membranes). A specific potential is applied to these outer membranes, electrochemically oxidizing and deactivating redox-active interferents like acetaminophen before they reach the inner sensing electrode. This approach has demonstrated a 72% reduction in redox-active interference [15].
- Carbon-Based Nanomaterials and Enzyme Engineering: Advances include using carbon nanomaterials (e.g., graphene, MXenes) to improve electrode conductivity and stability, coupled with chemical modification of GOx itself (mGOx) to enhance its performance and stability within the sensor [12].
Table 2: Research Reagent Solutions for Mitigating Acetaminophen Interference
| Research Reagent / Material | Function / Mechanism of Action | Key Findings / Performance |
|---|---|---|
| Cellulose Acetate | Hydrophobic polymer that creates a size-exclusion diffusion barrier. | Reduces access of larger interferent molecules to the electrode surface. Most effective when used in composites [3]. |
| Nafion | A sulfonated tetrafluoroethylene-based polymer. Creates a charged, permselective barrier that can repel acidic interferents. | The cellulose acetate/Nafion composite membrane effectively eliminated acetaminophen interference in an implantable sensor [3]. |
| Polyphenylenediamine (PPD) | An electrophymerized, non-conducting film deposited directly on the electrode. | Forms a dense film with tunable porosity that selectively removes ascorbic acid and other interferents [12]. |
| Gold-Coated Track-Etch Membranes | Conductive outer membrane. A potential is applied to electrochemically deactivate redox-active interferents before they reach the sensor. | Demonstrated a 72% reduction in redox-active interference and an 8-fold decrease in detection limit [15]. |
| MXene/GOx Polygel Nanocomposite (PGOx) | 2D MXene nanosheets provide a large surface area; polygels enhance enzyme stability. | Improves overall sensor stability and performance, which can indirectly improve selectivity. LOD of 3.1 μM for glucose [12]. |
Troubleshooting FAQs for Researchers
Q1: Our in-vitro sensor shows minimal interference, but significant discrepancy occurs in animal models. What could be the cause? A: This is a common issue. In-vitro tests often use buffers, while in-vivo, acetaminophen is metabolized. The primary metabolite in the interstitial fluid may be the actual interferent, not the parent compound. Implement an in-vivo microdialysis protocol [14] to directly measure the interferent profile in the ISF and correlate it with sensor error.
Q2: Why does the interference effect appear stronger at low glucose levels? A: The signal from the interferent is additive. At low glucose levels, the "background" current from glucose is small, so the fixed additional current from a given dose of acetaminophen constitutes a larger relative error, leading to a greater percentage overestimation of glucose [8].
Q3: We are using a Nafion coating, but interference persists. What are potential reasons? A: The thickness and morphology of the Nafion layer are critical. An overly thin or non-uniform coating may be incomplete. Consider using a composite membrane, such as cellulose acetate under Nafion, for a synergistic size-exclusion and charge-repulsion effect [3]. Also, validate that your working potential is optimized, as higher potentials exacerbate the issue.
Q4: Are there alternative sensing principles immune to acetaminophen interference? A: Yes. Second-generation sensors use redox mediators instead of Hâ‚‚Oâ‚‚ detection, often at lower operating potentials where acetaminophen is not oxidized. Third-generation sensors aim for direct electron transfer from the enzyme, also potentially avoiding this interference. Fluorescence-based sensors (e.g., Eversense) are inherently immune to electrochemical interferents [11] [12].
Acetaminophen interference in GOx-based sensors is a well-understood electrochemical phenomenon that remains a critical challenge for the development of robust implantable biosensors and the safe use of CGM systems. A deep understanding of the mechanism—competitive oxidation at the electrode surface—empowers researchers to select appropriate investigation protocols and implement effective mitigation strategies, such as advanced permselective membranes and novel conductive barriers. Continued research into these areas is essential for achieving the accuracy and reliability required for non-adjunctive glucose monitoring and closed-loop artificial pancreas systems.
Frequently Asked Questions (FAQs)
Q1: Why is acetaminophen a particularly common interferent for implantable biosensors? Acetaminophen is a significant interferent for first-generation electrochemical biosensors because it is easily oxidized at the working electrode's applied voltage. These sensors measure glucose by detecting an electrical current from the oxidation of hydrogen peroxide (Hâ‚‚Oâ‚‚), a byproduct of the glucose oxidase reaction. Acetaminophen competes in this reaction, generating an additional, non-glucose-related current that the sensor misinterpretes as a falsely high glucose concentration [8].
Q2: How does the route of administration (oral vs. intravenous) impact acetaminophen interference? The route of administration significantly affects the magnitude of interference. Intravenous (IV) administration can produce approximately twice the blood concentration of acetaminophen compared to oral intake. This higher concentration can lead to more severe and pronounced falsely elevated sensor readings. In one case, IV acetaminophen (15 mg/kg) caused a rapid spike in CGM readings, with an estimated discrepancy of 55 to 114 mg/dL compared to actual blood glucose, a larger effect than typically observed with oral doses [8].
Q3: What biosensor design features can help mitigate the effects of interferents like acetaminophen? Manufacturers incorporate several design features to reduce interference:
- Interference Membranes: Specialized permselective membranes are designed to limit the passage of common interfering substances to the working electrode [4].
- Bioprotective Membranes: These outer membranes provide biocompatibility and can also act as a barrier to interfering species [4].
- Nafion Coating: Applying a Nafion polymer coating to the electrode can improve sensor specificity by reducing the flux of interferents [16].
- Biosensor Generation: Second-generation biosensors use an artificial mediator, allowing for a lower operating potential that is less susceptible to oxidizing common interferents compared to first-generation (oxygen-dependent) systems [4].
Q4: Are there other common pharmaceutical substances known to interfere with biosensor performance? Yes, several other substances are known to cause interference, which is often detailed in manufacturer labeling. Common examples include:
- Ascorbic Acid (Vitamin C): Can falsely raise readings in some second-generation biosensor systems (e.g., FreeStyle Libre) [4].
- Hydroxyurea: Known to cause falsely elevated readings in first-generation systems from Dexcom and Medtronic [4].
- Salicylic Acid (in Aspirin): May slightly lower sensor glucose readings [4].
- Mannitol/Sorbitol: Can falsely elevate readings when administered intravenously [4].
- Tetracycline-class Antibiotics: May falsely lower sensor glucose readings in optical systems like the Senseonics Eversense [4].
Troubleshooting Guides
Guide 1: Diagnosing Unexplained Signal Spikes During In-Vitro Testing
Problem: During an in-vitro experiment with an implantable biosensor, you observe a rapid, unexplained increase in the sensor signal that does not correlate with the expected analyte concentration.
Possible Causes and Investigative Steps:
Review Recent Additives:
- Action: Immediately check the log of all pharmaceutical compounds or reagents introduced to the test solution.
- Investigation: Cross-reference these compounds against the known interferent list for your biosensor's technology (e.g., first-generation electrochemical). Acetaminophen, ascorbic acid, and hydroxyurea are primary suspects [4] [8].
Quantify the Interference:
- Action: If an interferent is suspected, design a controlled experiment to quantify its effect.
- Investigation: Spiked the test solution with the suspected interferent at a known, clinically relevant concentration while holding the glucose (or target analyte) concentration constant. Monitor the signal deviation. The table below summarizes quantitative interference data from a microneedle biosensor study, showing the relative impact of different substances [16]:
Table 1: Quantification of Interference on an Electrochemical Biosensor at 5 mM Glucose
| Interfering Substance | Concentration Tested | Interference (% Change in Signal) |
|---|---|---|
| Acetaminophen | Not Specified | Up to 150% |
| Ascorbic Acid | Not Specified | 4.5% - 17.8% |
| Urea | Not Specified | 4.2% - 11.3% |
- Verify Sensor Integrity:
- Action: Rule out sensor failure.
- Investigation: Perform a calibration check with a standard analyte solution free of any potential interferents. A stable and accurate reading suggests the spike was indeed due to chemical interference and not sensor drift.
Guide 2: Developing a Protocol for Systematic Interferent Screening
This guide provides a methodology for proactively evaluating the susceptibility of a biosensor to pharmaceutical interference, based on established practices in the field [4] [16].
Objective: To systematically test and quantify the effect of potential pharmaceutical interferents on the accuracy of an implantable biosensor in a controlled in-vitro environment.
Materials:
- Biosensor platform (e.g., functionalized microneedle array or other relevant design)
- Electrochemical analyzer (e.g., potentiostat)
- Standard analyte (e.g., D-glucose)
- Pharmaceutical interferents for testing (e.g., Acetaminophen, Ascorbic Acid, Hydroxyurea)
- Buffer solution (e.g., Phosphate Buffered Saline, PBS)
- Standard laboratory glassware and pipettes
Experimental Workflow:
Step-by-Step Protocol:
Baseline Establishment:
- Prepare a buffer solution with a known, physiologically relevant concentration of your target analyte (e.g., 5 mM glucose).
- Immerse the biosensor and measure the stable output signal. This is your baseline signal (I_glucose).
Interferent Introduction:
Signal Measurement and Analysis:
- Record the new sensor output (I_glucose+interferent).
- Calculate the percentage of signal interference using the formula:
- % Interference = [ (Iglucose+interferent - Iglucose) / I_glucose ] × 100
Data Compilation:
- Repeat steps 1-3 for all potential interferents and across a range of concentrations.
- Compile the results into an interference profile for the biosensor, similar to the example provided in Table 1.
Mitigation Strategy Testing:
- If a biosensor incorporates a special interference membrane or coating (e.g., Nafion), repeat the above protocol with and without the feature to quantitatively demonstrate its efficacy in reducing the interference effect [16].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Reagents for Interference Studies in Biosensor Research
| Research Reagent | Function in Experiment |
|---|---|
| Glucose Oxidase (GOx) | The biological recognition element immobilized on the working electrode. It catalyzes the oxidation of glucose, producing Hâ‚‚Oâ‚‚, which is measured to deduce glucose concentration [16]. |
| Nafion | A perfluorinated polymer used as an electrode coating. It acts as a permselective layer to reduce the flux of negatively charged interferents like ascorbic acid and uric acid to the electrode surface, improving specificity [16]. |
| Bovine Serum Albumin (BSA) & Glutaraldehyde | Used together as a cross-linking system to immobilize and stabilize the glucose oxidase enzyme on the electrode surface, preserving its activity [16]. |
| Acetaminophen (Paracetamol) | A critical reagent used as a positive control for interference testing, especially for first-generation electrochemical biosensors, due to its well-documented propensity to cause falsely elevated signals [8] [16]. |
| Ascorbic Acid | Another common positive control interferent for testing the selectivity of biosensors, particularly relevant for second-generation systems [4]. |
| Hydroxyurea | A pharmaceutical compound used in interference testing, known to cause significant positive interference in specific CGM systems [4]. |
| Egfr-IN-38 | Egfr-IN-38, MF:C25H24ClN7O2, MW:490.0 g/mol |
| Cdk7-IN-16 | CDK7 Inhibitor Cdk7-IN-16 |
Technical Support Center
Frequently Asked Questions (FAQs)
Q1: What is the mechanism by which acetaminophen interferes with continuous glucose monitors?
Acetaminophen interferes with the electrochemical sensing principle of many CGM systems. Most CGM devices use a first-generation electrochemical biosensor design that relies on glucose oxidase enzyme reactions. When interstitial glucose passes through the enzyme membrane, hydrogen peroxide (Hâ‚‚Oâ‚‚) is produced, which decomposes under applied voltage to release electrons that are converted into glucose readings [8]. However, acetaminophen's phenolic moiety is also oxidized at the electrode sensing surface, producing an additional electrochemical signal not related to glucose concentration [13] [8]. This results in falsely elevated CGM glucose values that do not correspond to actual blood glucose levels.
Q2: How does intravenous acetaminophen administration differ from oral administration in its interference effect?
Intravenous acetaminophen produces approximately twice the blood concentration of acetaminophen compared to oral administration and causes more significant CGM inaccuracies [8]. Clinical observations show IV administration (15 mg/kg over 15 minutes) causes a rapid increase in CGM readings, peaking at approximately 29.2 ± 1.9 minutes after administration, with estimated discrepancies of 55-114 mg/dL compared to capillary blood glucose measurements [8]. The intravenous route bypasses first-pass metabolism, leading to higher and more immediate serum concentrations that exacerbate the electrochemical interference effect.
Q3: Which CGM systems are most affected by acetaminophen interference?
First-generation electrochemical biosensor designs from Dexcom and Medtronic show significant susceptibility to acetaminophen interference [4]. Modern systems have implemented design improvements, but interference remains a concern. The specific affected models and their labeling are detailed in Table 1.
Q4: Why is acetaminophen interference particularly dangerous for Automated Insulin Delivery (AID) systems?
AID systems rely on accurate CGM readings to automatically calculate and deliver insulin doses. Falsely elevated CGM values can trigger unnecessary autocorrection boluses, potentially leading to dangerous hypoglycemic events [8]. This risk is amplified by the observation that acetaminophen interference produces greater discrepancies at lower blood glucose levels, creating a high-risk scenario where the system may administer insulin when actual glucose levels are already low or falling [8].
Q5: What design approaches are manufacturers implementing to reduce acetaminophen interference?
Manufacturers are employing multiple strategies to mitigate interference effects [4]:
- Permselective membranes: Designed to reduce the passage of interfering substances to the working electrode
- Multi-domain sensor designs: Incorporating interference membranes, bioprotective membranes, and diffusion resistance membranes
- Lower operating potentials: Newer systems reduce the applied voltage, minimizing oxidation of interfering substances
- Advanced biosensor generations: Movement toward second-generation (artificial mediator) and third-generation (direct electron transfer) designs
Troubleshooting Guides
Issue: Unexplained CGM Glucose Elevations Following Medication Administration
Symptoms: Rapid increase in CGM readings without corresponding blood glucose elevation; upward convex curve pattern; discrepancies lasting 2-8 hours.
Assessment Steps:
- Review recent medications: Identify any acetaminophen-containing products, including:
- Pain relievers (Tylenol)
- Cold and flu medications
- Fever reducers
- Note: Intravenous formulations pose highest risk [8]
Check CGM manufacturer specifications: Reference Table 1 for known interference patterns
Compare with blood glucose measurements: Conduct fingerstick testing to quantify discrepancy
Evaluate timing: Note that peak interference typically occurs 30 minutes-2 hours post-administration [13] [8]
Immediate Actions for AID Systems:
- Switch insulin pump to manual mode during acetaminophen effect window (2-8 hours post-administration) [8]
- Rely on blood glucose measurements rather than CGM values for treatment decisions
- Program temporary elevated glucose targets if system must remain in automated mode
- Increase blood glucose monitoring frequency during this period
Preventive Strategies:
- Identify acetaminophen-free alternative medications for pain/fever management
- Educate all healthcare providers about CGM interference risks
- Document interference events in patient records for future reference
- Consider CGM systems with lower acetaminophen susceptibility when available
Experimental Protocols for Interference Testing
In Vitro Assessment of Acetaminophen Interference
Objective: Quantify the effect of acetaminophen on CGM sensor accuracy under controlled conditions.
Materials:
- CGM sensors from multiple generations/manufacturers
- Acetaminophen stock solutions (varying concentrations)
- Glucose solutions spanning physiological range (40-400 mg/dL)
- Physiological buffer (pH 7.4)
- Electrochemical testing apparatus
- Statistical analysis software
Methodology:
- Sensor Calibration: Calibrate all sensors according to manufacturer specifications using glucose standards without interferents.
Interference Testing:
- Expose sensors to fixed glucose concentrations (100, 150, 200 mg/dL) while varying acetaminophen concentrations (0, 5, 10, 20 μg/mL)
- Measure sensor output at each combination
- Maintain constant temperature (37°C) and pH (7.4)
- Allow stabilization period between concentration changes
Data Collection:
- Record sensor readings at 1-minute intervals for 60 minutes
- Calculate mean absolute relative difference (MARD) for each condition
- Perform statistical analysis (ANOVA with post-hoc testing)
Dose-Response Characterization:
- Generate dose-response curves for acetaminophen interference
- Calculate interference magnitude as ΔGlucose = Sensor Reading - Actual Glucose
Clinical Validation Protocol
Objective: Evaluate acetaminophen interference in clinical setting with AID systems.
Study Design: Controlled crossover study with acetaminophen administration.
Participants: Type 1 diabetes patients using AID systems (n=20-40).
Intervention:
- Session 1: Oral acetaminophen (1000 mg)
- Session 2: Intravenous acetaminophen (15 mg/kg)
- Session 3: Placebo control
- Washout period: ≥7 days between sessions
Measurements:
- CGM readings every 5 minutes
- Reference blood glucose every 15 minutes (YSI or equivalent)
- Plasma acetaminophen levels at 0, 30, 60, 120, 240 minutes
- Insulin delivery data from AID system
Endpoint Analysis:
- Maximum CGM-blood glucose discrepancy
- Time to peak interference effect
- Duration of clinically significant interference (>20 mg/dL difference)
- Correlation between acetaminophen concentration and interference magnitude
Table 1: CGM System Interference Profiles and Manufacturer Labeling
| Manufacturer & Model | Biosensor Generation | Acetaminophen Interference | Other Labeled Interferents | Dose Consideration |
|---|---|---|---|---|
| Dexcom G6/G7/ONE/ONE+ | First-generation | Yes - may increase sensor readings | Hydroxyurea | >1000 mg every 6 hours in adults [4] |
| Medtronic Guardian 3/4/Sensor | First-generation | Yes - may falsely raise readings | Hydroxyurea | Any acetaminophen dose [4] |
| Medtronic Simplera | First-generation | Yes - may falsely raise sensor readings | Hydroxyurea | Medications containing acetaminophen [4] |
| Abbott FreeStyle Libre 2/3 | Second-generation | Not labeled | Ascorbic acid (Vitamin C) | >500 mg/day may affect readings [4] |
| Senseonics Eversense | Optical (Not electrochemical) | Not labeled | Tetracycline, Mannitol/Sorbitol | Unique interference profile [4] |
Table 2: Clinically Observed Acetaminophen Interference Magnitude
| Administration Route | Dose | Peak Discrepancy (mg/dL) | Time to Peak (minutes) | Duration of Effect | Study/Reference |
|---|---|---|---|---|---|
| Oral | 1000 mg | 21-30 mg/dL | 60-120 min | Up to 8 hours [13] | Maahs et al. (2015) [13] |
| Intravenous | 15 mg/kg | 55-114 mg/dL | 29.2 ± 1.9 min | >2 hours [8] | Matsuyama et al. (2025) [8] |
| Oral (G6 System) | 1000 mg | 3.1 ± 4.8 mg/dL | Not specified | Not specified | Manufacturer data [8] |
Signaling Pathways and Experimental Workflows
Diagram 1: Interference mechanism and testing workflow (Max Width: 760px)
Diagram 2: Clinical risk management protocol (Max Width: 760px)
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Interference Research
| Research Tool | Function/Application | Specifications/Considerations |
|---|---|---|
| First-generation CGM Sensors (Dexcom G6/G7, Medtronic Guardian) | Primary interference model systems; oxygen-dependent hydrogen peroxide detection | Use multiple lots; note membrane composition differences [4] |
| Second-generation CGM Sensors (Abbott FreeStyle Libre) | Control systems using artificial mediators; lower operating potential reduces interference [4] | Useful for comparative studies |
| Acetaminophen Reference Standards | Prepare precise concentrations for dose-response studies | Pharmaceutical grade; multiple solubility profiles (oral/IV simulations) [13] |
| Glucose Oxidase Enzyme | Understanding fundamental interference mechanism at molecular level | Multiple sources for reproducibility testing [8] |
| Electrochemical Testing Station | Controlled in vitro interference quantification | Capable of maintaining physiological temperature (37°C) and pH [17] |
| Physiological Buffer Systems | Simulate interstitial fluid environment for in vitro testing | pH 7.4; appropriate ionic composition [17] |
| HPLC/MS Equipment | Quantify acetaminophen concentrations in parallel with sensor testing | Validation of exposure concentrations [8] |
| Permselective Membrane Materials | Research on interference mitigation strategies | Various polymer compositions for flux control [4] |
| (R)-(+)-Pantoprazole-d6 | (R)-(+)-Pantoprazole-d6, MF:C16H15F2N3O4S, MW:389.4 g/mol | Chemical Reagent |
| MtTMPK-IN-4 | MtTMPK-IN-4|Inhibitor | MtTMPK-IN-4 is a potent M. tuberculosis thymidylate kinase inhibitor (IC50=6.1 µM). For Research Use Only. Not for human use. |
Engineering Solutions: Biosensor Designs and Methodologies to Counteract Interference
This technical support center is designed for researchers working on implantable biosensors, with a specific focus on mitigating acetaminophen (APAP) interference through advanced permselective membrane systems. The guidance below provides troubleshooting, experimental data, and validated protocols to support your development efforts.
Frequently Asked Questions (FAQs)
FAQ 1: What is the primary mechanism of acetaminophen interference in electrochemical biosensors? Acetaminophen interferes with first-generation electrochemical biosensors because it is an easily oxidizable substance. These sensors operate at a high voltage to measure the hydrogen peroxide (Hâ‚‚Oâ‚‚) produced from the glucose oxidase reaction. At this high potential, acetaminophen is also oxidized, generating a false additional current that is misinterpreted as higher glucose concentration [4] [8].
FAQ 2: How do permselective membranes function to exclude interferents? Permselective membranes are engineered domains within the sensor's layered architecture designed to be selectively permeable. They function as a physical and chemical barrier, strategically filtering molecules based on size, charge, or other properties before they reach the working electrode. This prevents electroactive interferents like acetaminophen from reacting at the electrode surface, while allowing glucose to pass through freely [4].
FAQ 3: Our in vitro interference screening shows promising results, but in vivo performance declines. What are potential causes? This common issue often relates to the biofouling process, which is not fully replicated in standard in vitro tests. After implantation, proteins and cells adhere to the sensor surface, forming a non-specific biofilm. This biofilm can alter the diffusion kinetics of both glucose and interferents, potentially reducing the effectiveness of the permselective membrane. Furthermore, the local inflammatory response can change the composition of the interstitial fluid, potentially concentrating interferents or creating new confounding factors [18]. Testing membranes with enhanced biocompatible coatings, such as specific hydrogels, may improve in vivo performance [18].
FAQ 4: Which biosensor generations are most susceptible to acetaminophen interference? Susceptibility varies by biosensor design generation [4]:
- First-Generation Biosensors (e.g., Dexcom G6/G7, Medtronic Guardian): Rely on oxygen and are highly susceptible to acetaminophen interference [4] [19].
- Second-Generation Biosensors (e.g., Abbott FreeStyle Libre): Use an artificial mediator and are less susceptible to acetaminophen but can be affected by high doses of ascorbic acid (Vitamin C) [4] [20].
- Third-Generation Biosensors (e.g., Sinocare iCan i3): Engineered for direct electron transfer and claim no interference from acetaminophen or vitamin C [4].
Troubleshooting Guides
Problem: Inconsistent interferent exclusion across sensor batches.
- Potential Cause 1: Inconsistent polymerization or cross-linking during membrane fabrication, leading to variations in pore size density.
- Solution: Implement more stringent quality control during polymer synthesis. Use techniques like scanning electron microscopy (SEM) to verify membrane morphology consistency. Ensure environmental conditions (temperature, humidity) are tightly controlled during fabrication.
- Potential Cause 2: Degradation of the polymer matrix during sterilization or storage.
- Solution: Evaluate alternative sterilization methods (e.g., gamma irradiation vs. ethylene oxide) and assess membrane performance post-sterilization. Optimize storage conditions to prevent polymer hydrolysis or oxidation.
Problem: Successful acetaminophen exclusion but significant oxygen limitation.
- Potential Cause: The permselective membrane's diffusion resistance to glucose is too low relative to oxygen, creating an oxygen-deficient environment around the glucose oxidase enzyme.
- Solution: Co-immobilize a custom oxygen reservoir material, such as perfluorocarbon, within the enzyme layer. Alternatively, engineer a composite membrane with a dedicated oxygen-rich domain to maintain a stable supply [4].
Experimental Data & Protocols
The following table summarizes quantitative data from a dynamic in vitro interference study, highlighting the response of different sensor types to various substances, including acetaminophen, at a stable glucose background of 200 mg/dL [19].
Table 1: Dynamic In-Vitro Interference Testing Results
| Substance Tested | Abbott Libre 2 (Max Bias) | Dexcom G6 (Max Bias) | Clinical Relevance Notes |
|---|---|---|---|
| Acetaminophen | No significant bias | > +100% | Confirmed key interferent for first-gen sensors [4] [19] |
| Ascorbic Acid | +48% | No significant bias | Key interferent for second-gen sensors [4] |
| N-Acetyl-Cysteine | +11% | +18% | Relevant as an APAP antidote [21] |
| Galactose | > +100% | +17% | Sugar alcohol potential interferent |
| Hydroxyurea | No significant bias | > +100% | Pharmaceutical interferent |
| Uric Acid | No significant bias | +33% | Relevant endogenous interferent |
Detailed Experimental Protocol: Chromatographic Analysis of APAP and Metabolites
This protocol allows for the simultaneous quantification of acetaminophen (APAP), its toxic metabolite NAPQI, and the antidote N-acetyl-cysteine (NAC) in plasma samples, useful for pharmacokinetic and toxicological studies [21].
1. HPLC Method for Simultaneous APAP, NAPQI, and NAC Analysis [21]
- Objective: To separate and quantify APAP, NAPQI, and NAC in a single analytical run.
- Equipment: HPLC system with a Photo-Diode Array (PDA) detector, Zorbax SB-C18 column (4.6 x 250 mm, 5 µm), pH meter, ultrasonic bath.
- Mobile Phase: Water, Methanol, and Formic acid in proportion (70:30:0.15, v/v/v).
- Flow Rate: 1.0 mL/min.
- Detection Wavelength: 254 nm.
- Injection Volume: 20 µL.
- Sample Preparation: Plasma samples should be protein-precipitated. Filter all samples through a 0.45 µm membrane filter before injection.
- Analysis: The total run time is approximately 5 minutes. Identify analytes by comparing retention times with pure standards.
2. HPTLC Method for Simultaneous APAP, NAPQI, and NAC Analysis [21]
- Objective: An alternative, cost-effective method for quantifying the same analytes.
- Equipment: HPTLC system with automatic sampler, silica gel 60 F254 plates (20 x 10 cm), twin-trough glass chamber, densitometer.
- Mobile Phase: Methanol, Ethyl Acetate, Glacial Acetic Acid (8:2:0.2, v/v/v).
- Sample Application: Apply samples as bands using an automatic sampler.
- Development: Saturate the chamber for 20 minutes. Allow mobile phase to ascend linearly to 9.0 cm.
- Detection: Air-dry plates and scan at 254 nm.
The Scientist's Toolkit
Table 2: Essential Research Reagents and Materials
| Item | Function / Explanation |
|---|---|
| N-Acetyl-Cysteine (NAC) | Used in studies as both a potential interferent and the primary antidote for APAP overdose; crucial for testing sensor specificity [21]. |
| Dithiothreitol (DTT) | A strong reducing agent used in interference studies; known to cause sensor fouling and failure, making it a stress-test agent for membrane robustness [19]. |
| Hydrogel Coatings | Polymers like polyethylene glycol (PEG) used to create a bioprotective domain; improve biocompatibility and reduce biofouling by creating a hydrophilic, protein-resistant surface [18]. |
| Permselective Polymers | Materials (e.g., polyurethanes, Nafion) used to form the interference domain; designed to be selectively permeable based on size and charge to exclude interferents [4]. |
| Glucose Oxidase | The core enzyme used in most CGM biosensors; catalyzes the oxidation of glucose, producing Hâ‚‚Oâ‚‚ that is measured electrochemically [4]. |
| Cdk1-IN-1 | Cdk1-IN-1|CDK1 Inhibitor|For Research Use Only |
| PROTAC IRAK4 degrader-2 | PROTAC IRAK4 degrader-2, MF:C57H68FN11O8S, MW:1086.3 g/mol |
Diagrams and Workflows
Biosensor Membrane Architecture
Dynamic Interference Testing Workflow
A significant challenge in the development and operation of continuous glucose monitoring (CGM) systems and other implantable biosensors is the distortion of signals caused by electroactive interfering substances, with acetaminophen being a primary culprit [4]. Accurate real-time monitoring is the cornerstone of effective diabetes management, especially for automated insulin delivery (AID) systems, which rely on precise sensor data to make dosing decisions [8]. Falsely elevated glucose readings due to acetaminophen interference can prompt these systems to deliver unneeded insulin, creating a serious risk of hypoglycemia for the user [8]. This technical support center document is designed to arm researchers and scientists with advanced electrochemical strategies, specifically the application of differential bias potentials, to identify, troubleshoot, and mitigate this critical interference in experimental settings.
Technical Background: Biosensor Design and Interference Mechanisms
Generations of Electrochemical Biosensors
Most commercially available CGM systems are based on first-generation electrochemical biosensor principles [4]. These sensors typically use glucose oxidase (GOx) as the recognition element. The core reaction involves the enzyme-catalyzed oxidation of glucose, which produces hydrogen peroxide (Hâ‚‚Oâ‚‚) [8]. The sensor then applies a specific bias potential to the working electrode, which causes the Hâ‚‚Oâ‚‚ to oxidize. This reaction releases electrons, generating a current that is proportional to the glucose concentration [8].
Second-generation systems, like certain Abbott FreeStyle Libre models, employ an artificial mediator to shuttle electrons, which can allow for operation at lower potentials [4]. Third-generation systems aim for direct electron transfer from the enzyme to the electrode [4]. Each design presents a distinct profile of susceptibility to interfering substances.
The Acetaminophen Interference Mechanism
Acetaminophen is an electroactive compound that can be readily oxidized at the electrode surface. Critically, the oxidation potential for acetaminophen often overlaps with that of hydrogen peroxide in first-generation biosensors [8]. When a standard, single potential is applied, the sensor's transducer cannot distinguish between the electrons generated from the glucose-correlated Hâ‚‚Oâ‚‚ and those from the oxidation of acetaminophen. The sensor interprets the total current as stemming from glucose, leading to a falsely elevated reported value [22] [8].
The following diagram illustrates this core interference mechanism at the sensor's electrode interface.
Interference Mechanism at the Electrode
Quantitative Impact of Acetaminophen Interference
The magnitude of interference is dependent on dosage, route of administration, and the specific sensor design. The following table summarizes documented discrepancies across different scenarios.
Table 1: Documented Acetaminophen Interference Effects
| CGM Model | Acetaminophen Dose & Route | Observed Effect on Sensor Glucose | Reference |
|---|---|---|---|
| Guardian 4 (Medtronic) | 15 mg/kg (IV) | Peak discrepancy of 55-114 mg/dL vs. blood glucose; peak at ~30 min post-dose. | [8] |
| Dexcom G6 | 1 g (Oral) | Mean increase in discrepancy of 3.1 mg/dL (±4.8 mg/dL) vs. plasma glucose. | [8] |
| Older Guardian REAL-TIME | 1 g (Oral) | Increase of ~21 mg/dL from baseline (at plasma glucose ~90 mg/dL). | [8] |
| Dexcom G4 Platinum | 1 g (Oral) | Increase of ~30 mg/dL from baseline (at plasma glucose ~90 mg/dL). | [8] |
| General Trend | N/A | Discrepancy is significantly greater at lower blood glucose levels. | [8] |
Troubleshooting Guides & FAQs
This section addresses common experimental and technical challenges.
FAQ 1: Why does our lab-built biosensor show high background noise and signal drift in complex biological fluids like undiluted serum?
Answer: This is a classic symptom of biofouling and non-specific binding (NSB). Proteins and other biomolecules in the sample can adsorb onto the electrode surface, forming an insulating layer that increases charge-transfer resistance and degrades signal stability [23].
- Mitigation Strategies:
- Surface Passivation: Implement a robust permselective membrane. Common materials include Nafion (which is also cation-selective) or chitosan-based biopolymer films [4] [24]. These membranes create a physical and chemical barrier that limits the access of large, potentially interfering molecules to the electrode surface while allowing smaller analytes like Hâ‚‚Oâ‚‚ to pass.
- Hydrogel Layers: Incorporate a bioprotective hydrogel membrane (e.g., based on cross-linked poly(2-hydroxyethyl methacrylate)) [4]. This layer is designed to be biocompatible and to resist protein adhesion, thereby reducing biofouling over extended implantation periods.
- Low-Fouling Coatings: Modify the electrode surface with hydrophilic polymers like polyethylene glycol (PEG) or zwitterionic materials, which are highly resistant to protein adsorption.
FAQ 2: Our sensor successfully discriminates against acetaminophen in buffer, but performance degrades drastically in whole blood. What could be the cause?
Answer: This indicates that the interference rejection strategy is insufficient for real-world matrices. Whole blood contains a complex cocktail of electroactive interferents beyond acetaminophen, such as ascorbic acid (Vitamin C), uric acid, and lactate, which may oxidize at similar potentials [22] [20]. Your sensor's selectivity layer might be optimized for a single interferent but not for a complex mixture.
- Troubleshooting Steps:
- Systematic Interference Screening: Test your sensor not only with acetaminophen but also with a panel of common physiological interferents (ascorbic acid, uric acid, dopamine) both individually and in combination.
- Optimize the Interference Membrane: Re-evaluate the composition and thickness of your permselective membrane. A multi-domain membrane structure, as used in commercial sensors (e.g., Dexcom G6/G7), may be necessary to effectively screen out multiple classes of interfering molecules [4].
- Explore Advanced Materials: Consider incorporating molecularly imprinted polymers (MIPs) designed to have specific cavities for acetaminophen, thereby trapping it before it reaches the electrode. Alternatively, the use of metallic nanoparticles or metal-organic frameworks (MOFs) can catalyze the specific oxidation of Hâ‚‚Oâ‚‚ at a lower potential, widening the window for discrimination [25].
FAQ 3: When testing differential potentials, how do we determine the optimal secondary potential for accurate signal discrimination?
Answer: Identifying the optimal secondary potential requires a systematic characterization of the current-potential (I-V) profiles for both your target analyte (Hâ‚‚Oâ‚‚, correlated to glucose) and the primary interferent (acetaminophen).
- Experimental Protocol:
- Cyclic Voltammetry (CV) Scans: Perform CV in a standard solution containing only your electrolyte (e.g., PBS). This is your background.
- Analyte-Specific CV: Add a known concentration of Hâ‚‚Oâ‚‚ to the solution and run CV again. Identify the potential (or potential range) where the Hâ‚‚Oâ‚‚ oxidation current is maximal and stable.
- Interferent-Specific CV: In a fresh cell, run CV with a physiologically relevant concentration of acetaminophen. Identify its oxidation peak potential.
- Identify the "Silent" Window: Analyze the voltammograms to find a potential where the oxidation current for acetaminophen is minimal (i.e., it is not being oxidized), while a measurable, stable background current for Hâ‚‚Oâ‚‚ is still maintained. This potential often lies between the oxidation peaks of the two species or at a value below the interferent's oxidation onset.
- Validation: Calibrate your sensor using both the primary (measuring) potential and the secondary (discrimination) potential with solutions containing glucose only, acetaminophen only, and a mixture of both.
Experimental Protocols for Signal Discrimination
Protocol: Dual-Potential Amperometry for Acetaminophen Rejection
This protocol outlines a method to operate a biosensor at two different bias potentials to mathematically correct for acetaminophen interference.
Principle: The sensor is operated by rapidly toggling between a primary measuring potential and a secondary discrimination potential. The current at the primary potential contains signal from both glucose and acetaminophen. The current at the secondary potential is designed to be sensitive mainly to acetaminophen. A correction algorithm then subtracts the interferent contribution.
Workflow:
Dual-Potential Amperometry Workflow
Materials:
- Potentiostat/Galvanostat
- Custom-built or commercial 3-electrode system (WE: Pt; RE: Ag/AgCl; CE: Pt)
- GOx-modified working electrode
- Phosphate Buffered Saline (PBS), pH 7.4
- D-(+)-Glucose stock solution
- Acetaminophen stock solution
Procedure:
- Electrode Calibration: Immerse the sensor in stirred PBS at 37°C. Apply the primary potential (e.g., +0.6 V vs. Ag/AgCl) and record the background current until stable.
- Glucose Response: Add aliquots of glucose stock solution to achieve a range of known concentrations (e.g., 50-400 mg/dL). Record the steady-state current at the primary potential (IglucoseV1) for each concentration.
- Interferent Calibration at V1: Return to a baseline glucose level. Add acetaminophen to a clinically relevant concentration (e.g., 10-100 µM). Record the current increase at V1 (IacetV1).
- Identify Secondary Potential (V2): Using CV as described in FAQ 3, select a potential (V2, e.g., +0.3 V vs. Ag/AgCl) where Hâ‚‚Oâ‚‚ oxidation is minimal but acetaminophen still generates a significant current.
- Calibrate Interferent Response at V2: With acetaminophen present, switch the applied potential to V2 and record the steady-state current (IacetV2). Establish a correlation factor (k) between IacetV1 and IacetV2.
- Dual-Potential Operation & Validation: In a new solution containing both glucose and acetaminophen, rapidly pulse the applied potential between V1 and V2. Record ItotalV1 and IacetV2.
- Data Processing: Calculate the corrected glucose current: Icorrected = ItotalV1 - (k * IacetV2). Use the calibration curve from Step 2 to convert Icorrected to a glucose concentration.
The Scientist's Toolkit: Key Research Reagent Solutions
Table 2: Essential Materials for Biosensor Interference Research
| Reagent/Material | Function in Experimentation | Example & Notes |
|---|---|---|
| Glucose Oxidase (GOx) | Biological recognition element; catalyzes glucose oxidation. | From Aspergillus niger. Ensure high specific activity (>200 U/mg). |
| Permselective Membranes | Reduces flux of interferents; enhances selectivity. | Nafion (cation exchanger), Poly-o-phenylenediamine (electropolymerized), Chitosan-based films [24]. |
| Bioprotective Membranes | Prevents biofouling; improves in vivo biocompatibility and longevity. | Cross-linked hydrogels (e.g., poly-HEMA). |
| Electrochemical Cell | Provides controlled environment for 3-electrode measurements. | Custom cell or commercial vessel (e.g., from Metrohm, BASi). |
| Potentiostat | Applies potential and measures resulting current. | Essential for amperometry, CV, and EIS. |
| Artificial Interstitial Fluid | Physiologically relevant testing matrix. | Contains key electrolytes (Na+, K+, Cl-, Ca2+) at physiological levels and pH (7.3-7.4). |
| Fak-IN-5 | Fak-IN-5, MF:C29H29ClF3N3O4, MW:576.0 g/mol | Chemical Reagent |
| Hdac-IN-38 | HDAC-IN-38|HDAC Inhibitor|For Research Use | HDAC-IN-38 is a potent HDAC inhibitor for neuroscience research. It improves cerebral blood flow and cognitive function. This product is for research use only, not for human consumption. |
Advanced Experimental & Data Analysis Techniques
Protocol: Electrochemical Impedance Spectroscopy (EIS) for Sensor Characterization
EIS is a powerful, non-destructive method for probing the interfacial properties of a modified electrode, which is crucial for diagnosing issues like biofouling or ineffective membrane deposition [23].
Principle: A small amplitude AC potential is applied across a range of frequencies, and the complex impedance (Z) of the system is measured. The data is often presented as a Nyquist plot.
Procedure:
- Setup: Configure the potentiostat for EIS mode. Set the DC potential to your sensor's operating point (e.g., +0.6 V). Set the AC voltage amplitude to 10 mV. Scan frequencies from 100 kHz to 0.1 Hz.
- Baseline Measurement: Run EIS on your bare or modified electrode in a clean electrolyte solution.
- Post-Modification Measurement: After applying a membrane (e.g., Nafion), run EIS again in the same solution. A successful coating will typically increase the charge-transfer resistance (Rct), visible as a larger diameter of the semicircle in the Nyquist plot.
- Post-Exposure Measurement: After exposing the sensor to a complex fluid (e.g., serum) or an interferent, run EIS again. An increase in Rct compared to the baseline often indicates successful fouling or binding events, which can be correlated with performance degradation.
Data Analysis: Machine Learning for Enhanced Discrimination
For complex datasets generated from multi-potential or multi-analyte experiments, machine learning (ML) can be a powerful tool to deconvolute signals.
- Approach: Use the currents from multiple applied potentials (not just two) as input features for a regression model (e.g., Random Forest, Support Vector Regression).
- Process:
- Feature Collection: For each sample, collect steady-state currents or integrated charge from a sequence of pulses to different potentials.
- Training Set: Create a large training set with known concentrations of glucose, acetaminophen, and other interferents, both alone and in mixtures.
- Model Training: Train the ML model to predict the reference glucose concentration based on the multi-potential current data.
- Validation: Test the model on a separate validation set not used in training. A well-trained model can effectively learn the unique "fingerprint" of each species' electrochemical behavior and provide a more robust glucose prediction in the presence of complex interference.
For researchers developing implantable biosensors, achieving accurate and selective measurements in complex biological matrices remains a significant challenge. A primary obstacle is signal interference from electroactive compounds that co-exist with the target analyte. Acetaminophen (APAP or paracetamol), a widely used over-the-counter analgesic and antipyretic, is a particularly problematic interferent for continuous glucose monitoring (CGM) systems and other implantable biosensors [4] [26].
This interference arises because acetaminophen oxidizes at electrochemical potentials that can overlap with those of target analytes, leading to falsely elevated readings [4] [27]. The consequences are not merely academic; they directly impact patient safety. For example, manufacturer labeling for leading CGM systems explicitly warns that taking higher than maximum dosage of acetaminophen (e.g., >1000 mg every 6 hours in adults) may falsely increase sensor glucose readings [4]. In the context of drug development and biomedical research, such interference can compromise data integrity and therapeutic monitoring.
This technical support article explores how biomimetic catalysts and nanozymes—nanomaterials with enzyme-like properties—offer innovative pathways to overcome these selectivity challenges. By providing alternative sensing mechanisms with enhanced specificity, these advanced materials present promising solutions for next-generation biosensing platforms where traditional enzymes fall short.
Frequently Asked Questions (FAQs)
Q1: Why is acetaminophen such a prevalent interferent in electrochemical biosensors?
Acetaminophen's electrochemical behavior makes it particularly problematic. Its phenol group undergoes a two-electron, two-proton oxidation to form N-acetyl-p-benzoquinoneimine (NAPQI) at potentials that often overlap with those required to detect other important biomarkers [27]. This oxidation reaction is pH-dependent, with peak potential shifting to less positive values as pH increases [27]. In complex biological samples like serum, saliva, or interstitial fluid, acetaminophen can be present at therapeutic concentrations (0.01–0.05 mg/mL in saliva) [28], creating significant interference challenges for biosensors monitoring glucose, neurotransmitters, or other metabolites.
Q2: How do first-generation biosensors differ from later generations in their susceptibility to interference?
Biosensor generations are classified by their electron transfer mechanisms, which directly impact interference susceptibility:
- First-generation systems employ oxygen as a natural electron shuttle and typically require high operating potentials, making them highly susceptible to interference from electroactive compounds like acetaminophen and ascorbic acid [4] [26].
- Second-generation systems use artificial mediators to lower the required overpotential, thereby reducing interference from compounds that oxidize at higher potentials [26].
- Third-generation systems facilitate direct electron transfer between the enzyme and electrode, operating at near-physiological potentials where fewer interfering compounds are electroactive [26].
Q3: What advantages do nanozymes offer over natural enzymes in biosensing applications?
Nanozymes provide several distinct advantages that make them attractive for biosensing:
- Superior stability: They maintain catalytic activity under a wider range of temperature and pH conditions compared to natural enzymes [29] [30].
- Tunable activity: Their catalytic properties can be precisely engineered through surface modification, doping, or morphology control [31] [30].
- Cost-effectiveness: They can be produced at lower cost with more consistent quality than purified natural enzymes [29].
- Multi-enzyme mimicry: Single nanozyme systems can exhibit multiple enzyme-like activities, enabling complex cascade reactions [30].
Q4: What design strategies can improve the selectivity of biomimetic sensors?
Several innovative design strategies can enhance sensor selectivity:
- Biomimetic active sites: Designing nanozymes with atomic structures that mimic natural enzyme active sites (e.g., Fe-Nâ‚„ coordination) improves substrate specificity [30].
- Surface functionalization: Adding specific functional groups (e.g., phenolic hydroxyl groups) can create hydrogen-bonding sites that preferentially capture target molecules [32].
- Stimuli-responsive design: Creating nanozymes that activate only in response to specific microenvironmental cues (pH, redox conditions) enhances selective operation in complex biological environments [30].
Troubleshooting Guide: Common Experimental Challenges
| Challenge | Possible Causes | Solutions & Recommendations |
|---|---|---|
| Poor Selectivity | - Non-specific binding- Overlapping oxidation potentials- Inadequate membrane barrier | - Incorporate permselective membranes (Nafion/cellulose acetate) [26]- Use sentinel sensors for background subtraction [26]- Implement multi-sensor arrays with chemometrics [26] |
| Signal Drift | - Biofouling in biological matrices- Enzyme/nanozyme instability- Reference electrode potential shift | - Apply anti-biofouling coatings (bioprotective membranes) [4]- Optimize immobilization techniques- Use more stable nanozyme materials [30] |
| Low Sensitivity | - Poor electron transfer kinetics- Suboptimal operating potential- Insufficient active sites | - Incorporate conductive nanomaterials (graphene, CNTs) [31] [32]- Fine-tune applied potential using CV- Increase effective surface area with porous structures [32] |
| Inconsistent Performance | - Batch-to-batch nanozyme variation- Uneven modification of electrode surface- Uncontrolled microenvironment | - Standardize synthesis protocols [29]- Implement precise deposition methods (inkjet printing)- Control immobilization matrix carefully |
Experimental Protocols: Methodologies for Advanced Sensing
Protocol 1: Development of Metal-Organic Framework (MOF)-Based Acetaminophen Sensor
This protocol details the construction of an ultrasensitive APAP sensor using hydroxylated Fe-MOF and carbon nanofibers, achieving a detection limit of 27.81 nM with high anti-interference capability [32].
Materials & Reagents:
- Fe-DOBDC (phenol-hydroxyl-functionalized Fe-MOF)
- Carbon nanofibers (CNF)
- N,N-dimethylformamide (DMF)
- Ethanol
- Phosphate buffer saline (PBS, 0.1 M, pH 7.4)
- Acetaminophen standard
- Glassy carbon electrode (GCE)
Procedure:
- Synthesis of Fe-DOBDC: Solvothermally synthesize phenol-hydroxyl-functionalized Fe-MOF using 2,5-dihydroxyterephthalic acid (DOBDC) as the ligand and iron salt as the metal precursor [32].
- Composite Preparation: Mechanically grind Fe-DOBDC with carbon nanofibers (CNF) in a mass ratio of 5:1 to form an interlaced 3D "electronic spider web" architecture [32].
- Electrode Modification: Disperse 2 mg of Fe-DOBDC/CNF composite in 1 mL ethanol by sonication for 30 minutes. Deposit 5 μL of the suspension onto a polished GCE and dry under infrared light [32].
- Electrochemical Measurement: Perform differential pulse voltammetry (DPV) in 0.1 M PBS (pH 7.4) with potential range of 0.2-0.6 V vs. Ag/AgCl. The sensor exhibits bilinear responses in the ranges of 0.09-20 μM and 20-450 μM [32].
Validation:
- Test sensor performance in artificial saliva or serum samples
- Evaluate recovery rates (expected: 95.93-103.4%)
- Assess interference from common electroactive compounds (ascorbic acid, uric acid, glucose)
- Test long-term stability (94.33% signal retention after 11 days) [32]
Protocol 2: Smartphone-Integrated Biosensor for Salivary Acetaminophen Monitoring
This protocol describes a point-of-care approach for non-invasive APAP monitoring using smartphone-based detection, applicable for therapeutic drug monitoring (0.01-0.05 mg/mL range) [28].
Materials & Reagents:
- Artificial saliva (pH 6.5-7.0)
- Paracetamol (acetaminophen) standard
- KickStat potentiostat or similar portable electrochemical device
- Screen-printed carbon electrodes (SPCE)
- MediMeter smartphone application (or similar custom-developed app)
Procedure:
- Sensor Preparation: Modify screen-printed carbon electrodes with suitable recognition element (enzyme-based or nanozyme-based) [28].
- Sample Collection & Preparation: Collect saliva samples and centrifuge at 10,000 × g for 5 minutes to remove particulate matter. For initial validation, use artificial saliva spiked with known APAP concentrations [28].
- Electrochemical Measurement: Using the KickStat potentiostat connected to a smartphone, perform chronoamperometric measurements at an optimized potential (typically +0.4 - +0.5 V vs. Ag/AgCl) [28].
- Data Analysis: The MediMeter application automatically converts current signals to concentration values using a pre-calibrated standard curve (R² = 0.988 for electrochemical method) [28].
Method Comparison:
- Colorimetric method: Simpler instrumentation but lower precision (R² = 0.939)
- Electrochemical method: Better precision and faster response (~1 minute) but requires more expensive equipment [28]
Protocol 3: Biomimetic Nanozyme Sensor with Stimuli-Responsive Properties
This advanced protocol utilizes intelligent nanozymes whose catalytic activities can be modulated by specific stimuli, enabling highly selective detection in complex environments [30].
Materials & Reagents:
- Single-atom nanozymes (e.g., Fe-N-C, Zn-N-C)
- Stimuli-responsive polymers (pH-, light-, or temperature-sensitive)
- Target-specific ligands for functionalization
- Buffer solutions at various pH values
Procedure:
- Nanozyme Design: Synthesize single-atom nanozymes with specific coordination environments (e.g., Fe-Nâ‚„) that mimic natural enzyme active sites [30].
- Surface Functionalization: Modify nanozymes with stimuli-responsive elements that activate only under specific conditions (e.g., acidic pH in inflammatory environments) [30].
- Sensor Fabrication: Immobilize functionalized nanozymes on electrode surfaces using appropriate cross-linking strategies.
- Stimuli-Responsive Testing: Characterize sensor performance under different environmental conditions to validate selective activation/deactivation of catalytic activity [30].
Key Considerations:
- Precise control of coordination environment is crucial for enzyme-like activity
- Stimuli-responsive elements should be chosen based on target application environment
- Comprehensive kinetic studies (KM, Vmax) should be performed to compare with natural enzymes [30]
Signaling Pathways & Experimental Workflows
Diagram 1: Conceptual roadmap illustrating the evolution from recognizing acetaminophen interference to developing nanozyme-based solutions through different biosensor generations and advanced material designs.
Diagram 2: Acetaminophen interference mechanisms and corresponding mitigation strategies, showing the relationship between the fundamental problems and practical solutions in biosensor design.
Research Reagent Solutions
Table 1: Essential materials for developing biomimetic and nanozyme-based sensors
| Research Reagent | Function & Application | Key Characteristics |
|---|---|---|
| Single-Atom Nanozymes (Fe-N-C) [30] | Mimic metalloenzyme active sites for oxidoreductase-like activity | Precisely defined coordination structure (e.g., Fe-Nâ‚„), high catalytic activity, good stability |
| Hydroxylated Fe-MOFs (Fe-DOBDC) [32] | Hydrogen-bond mediated specific recognition of acetaminophen | Phenolic hydroxyl groups for specific binding, high surface area, tunable porosity |
| Carbon Nanofibers (CNF) [32] | Conductive network formation in composite sensors | High conductivity, one-dimensional structure, mechanical flexibility |
| Metallophthalocyanines (MPc) [31] | Biomimetic catalysts for electrochemical sensing | Nâ‚„ macrocyclic structure, excellent redox activity, structural tunability |
| PEDOT-Graphene Nanocomposites [31] | Enhanced electron transfer in sensing interfaces | High conductivity, stability, synergistic effects with biomimetic catalysts |
| Stimuli-Responsive Polymers [30] | Enable "smart" nanozymes with activatable catalysis | Respond to pH, light, temperature, or specific biomarkers |
The integration of biomimetic and nanozyme catalysts represents a paradigm shift in addressing the persistent challenge of acetaminophen interference in implantable biosensors. By moving beyond traditional enzyme-based designs, researchers can leverage the tunable catalytic properties, enhanced stability, and biomimetic specificity of these advanced materials to create more reliable sensing platforms. The experimental protocols and troubleshooting guidance provided in this technical resource offer practical pathways for implementing these innovative solutions, ultimately contributing to the development of more accurate and clinically viable biosensors for therapeutic monitoring and diagnostic applications.
Acetaminophen is a well-documented interferent in continuous glucose monitoring (CGM) systems due to its electroactive properties, which can cause falsely elevated glucose readings through oxidation at the sensor's working electrode [4] [8]. This interference presents a significant challenge for implantable biosensors, particularly for patients requiring both glucose monitoring and pain management, as inaccurate readings could trigger inappropriate therapeutic responses in automated insulin delivery systems [8]. Material innovations in electrode modifications and functional layers represent the forefront of research aimed at mitigating these confounding signals while maintaining accurate target analyte detection.
Mechanisms of Acetaminophen Interference
Electrochemical biosensors for glucose typically utilize glucose oxidase (GOx) as the recognition element. In first-generation biosensor designs, the enzymatic reaction produces hydrogen peroxide (Hâ‚‚Oâ‚‚), which is oxidized at the working electrode under an applied voltage, generating an electrical current proportional to glucose concentration [4] [8]. Acetaminophen interferes because it is also readily oxidized at similar potentials, contributing additional current that the sensor misinterpretes as glucose [8].
This interference mechanism is particularly problematic for implantable sensors operating in complex biological environments where multiple electroactive species coexist. The table below summarizes the documented effects of acetaminophen interference across different commercial CGM systems:
Table 1: Documented Acetaminophen Interference in Commercial CGM Systems
| CGM Manufacturer and Model | Biosensor Generation | Reported Acetaminophen Interference Effect |
|---|---|---|
| Dexcom G6/G7, Dexcom ONE/ONE+ [4] | First | Taking higher than maximum dosage (>1000 mg every 6 hours in adults) may increase sensor readings |
| Medtronic Guardian Connect/Simplera [4] | First | May falsely raise sensor glucose readings; level of inaccuracy depends on acetaminophen levels |
| Abbott FreeStyle Libre series [22] | Second | Primary interferent is ascorbic acid (Vitamin C); acetaminophen not specifically listed as key interferent |
Diagram: Acetaminophen interference mechanism at the biosensor electrode. The interferent bypasses the enzyme layer and is directly oxidized, contributing to the measured current.
Material Solutions for Interference Mitigation
Membrane-Based Exclusion Strategies
A primary approach involves engineering sophisticated membrane architectures that control the flux of molecules reaching the electrode surface. Leading CGM manufacturers incorporate multiple functional membranes or "domains" around the working electrode, including:
- Interference membranes designed to reduce the passage of interfering species like acetaminophen [4]
- Bioprotective membranes providing biocompatibility while also influencing interferent passage [4]
- Diffusion resistance membranes that control the relative flux of glucose versus oxygen [4]
Recent research has demonstrated a novel conductive membrane encapsulation strategy that electrochemically deactivates redox-active interferents while allowing redox-inactive target analytes to pass through unaltered. In one study, this approach utilizing three layers of gold-coated track-etch membranes achieved a 72% reduction in redox-active interference and an 8-fold decrease in detection limit [15].
Advanced Sensing Materials and Mediators
Second-generation biosensors employ artificial mediator species instead of oxygen, allowing operation at reduced potentials where acetaminophen is less likely to oxidize [4]. The Abbott FreeStyle Libre systems utilize this design, which correspondingly shifts their primary interference profile from acetaminophen to ascorbic acid [4] [22].
Third-generation systems represent the next evolutionary step, engineered for direct electron transfer between the enzyme cofactor and electrode surface without mediators. The Sinocare iCan i3 CGM system exemplifies this design, with manufacturer claims of no acetaminophen or vitamin C interference [4].
Emerging research explores redox-active metal-organic frameworks (MOFs) modified with mediator materials that act as "wires" for efficient electron exchange between enzymes and electrodes. This strategy enhances both reaction efficiency and long-term stability while potentially reducing interference vulnerability [33].
Experimental Protocols for Validation
In Vitro Interference Testing Protocol
Researchers evaluating new electrode modifications should implement rigorous interference testing using this standardized protocol:
Sensor Preparation: Fabricate sensors with the proposed functional layers/membranes. Ensure consistent membrane thickness and composition across test groups.
Baseline Measurement: Immerse sensors in PBS solution (pH 7.4) at 37°C. Apply operating potential and record baseline current until stable (±5% over 10 minutes).
Glucose Response Calibration: Add glucose aliquots to create concentrations spanning the physiological range (2-30 mM). Record current response at each concentration to establish glucose sensitivity.
Interferent Challenge: To the calibrated system, introduce acetaminophen at therapeutic concentrations (0.05-0.30 mM). Monitor current response for 60 minutes.
Data Analysis: Calculate the apparent glucose equivalent using the previously established glucose sensitivity: Apparent Glucose (mM) = ΔI (after acetaminophen) / Glucose Sensitivity (nA/mM)
Specificity Assessment: Repeat with other common interferents (ascorbic acid, uric acid) to assess selectivity.
Conductive Membrane Protection Protocol
For researchers implementing the conductive membrane strategy [15]:
Membrane Fabrication: Prepare track-etch membranes (polycarbonate, 10-30 μm thickness) and deposit gold coating via sputtering (30-100 nm thickness).
Sensor Encapsulation: Assemble the conductive membrane layers around a conventional glucose oxidase sensor, ensuring electrical contact for potential application.
Potential Optimization: Apply sweeping potentials (0-0.4V vs. Ag/AgCl) to the conductive membrane to determine the optimal interference oxidation potential.
Performance Validation: Test sensor response to glucose (2-20 mM) with and without acetaminophen (0.20 mM) present, comparing signal-to-noise ratios.
Troubleshooting Guide: FAQs
Table 2: Troubleshooting Common Experimental Challenges
| Problem | Potential Cause | Solution |
|---|---|---|
| Incomplete interference rejection | Membrane porosity too high/conductive membrane potential suboptimal | Optimize membrane pore size (MWCO 100-500 Da); systematically tune applied potential to conductive membrane [15] |
| Reduced glucose sensitivity after modification | Excessive diffusion barrier from thick functional layers | Implement thinner, more selective layers; explore facilitated transport materials [4] |
| Signal drift during long-term testing | Biofouling or mediator leaching | Incorporate bioprotective membranes; cross-link mediators to prevent leaching [4] [33] |
| Inconsistent performance between batches | Variations in membrane deposition/electrode fabrication | Standardize fabrication protocols with quality control checkpoints for thickness and composition |
Q: Why does acetaminophen interference appear greater at lower glucose levels in clinical observations?
A: This phenomenon, documented in a case study with intravenous acetaminophen [8], likely stems from the relative contribution of the interference signal. At lower glucose levels, the acetaminophen oxidation current represents a larger percentage of the total signal, creating a proportionally greater discrepancy. Material solutions must therefore demonstrate efficacy across the entire physiological glucose range.
Q: How can researchers balance interference rejection with maintaining rapid glucose response times?
A: This fundamental trade-off requires optimizing membrane hydrophilicity/hydrophobicity balance and incorporating nanoscale transport channels. Materials like thin hydrogel layers with molecularly imprinted polymers show promise for selectively retarding interferents while permitting relatively unimpeded glucose diffusion [4] [15].
Q: What validation benchmarks should new materials meet before progressing to in vivo testing?
A: Minimum benchmarks include: <5% signal contribution from therapeutic acetaminophen concentrations (0.20 mM), >90% retained glucose sensitivity after 72-hour continuous operation, and stability across physiological pH (7.0-7.6) and temperature (35-39°C) variations [4] [8].
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for Interference Mitigation Research
| Research Material | Function/Application | Key Considerations |
|---|---|---|
| Track-etch membranes (polycarbonate) [15] | Substrate for conductive interference rejection layers | Tunable pore size (0.01-1.0 μm); enables controlled flux of analytes and interferents |
| Gold sputtering targets [15] | Creating conductive membrane surfaces | Enables application of electrostatic potentials to oxidize interferents before they reach sensing electrode |
| Glucose oxidase (GOx) [4] | Primary recognition element for glucose sensing | Immobilization method (entrapment, cross-linking, covalent binding) critically impacts stability |
| Metal-organic frameworks (MOFs) with redox mediators [33] | Enhancing electron transfer efficiency | Modified MOFs act as "molecular wires" between enzyme active sites and electrodes |
| Permselective polymers (e.g., Nafion, polypyrrole) [4] | Charge-based exclusion of interferents | Effectiveness depends on interferent charge at physiological pH; may require composite formulations |
| Cross-linking agents (e.g., glutaraldehyde) [4] | Stabilizing enzyme and membrane layers | Concentration and reaction time must be optimized to prevent compromised activity |
| TbPTR1 inhibitor 1 | TbPTR1 Inhibitor 1 | TbPTR1 Inhibitor 1 targets pteridine reductase for trypanosome research. This product is For Research Use Only. Not for human or veterinary diagnostic or therapeutic use. |
| Ret-IN-10 | Ret-IN-10, MF:C29H28N8OS, MW:536.7 g/mol | Chemical Reagent |
Diagram: Multi-layer protection strategy combining conductive and permselective membranes.
Future Directions
The next frontier in interference mitigation involves smart materials that adapt to their chemical environment, potentially using molecularly imprinted polymers with affinity for specific interferents or stimuli-responsive membranes that change permeability in the presence of confounding species. Additionally, the integration of artificial intelligence for signal processing can complement material innovations by identifying characteristic interference patterns in the sensor data, creating a multi-layered defense against acetaminophen and other clinically relevant interferents [34]. As these technologies mature, they will enable increasingly accurate and reliable implantable biosensors capable of operating effectively in the complex chemical environment of the human body.
Frequently Asked Questions (FAQs)
Q1: What are the most effective biocompatible coatings for reducing acetaminophen interference in implantable glucose biosensors? A1: Nafion is a highly effective perfluorinated polymer coating that creates a charge-based barrier, significantly reducing the flux of negatively charged interferents like acetaminophen to the sensor's working electrode [16]. Phosphorylcholine (PC)-based coatings mimic cell membranes and are also used to reduce non-specific adsorption and improve the biocompatibility of implanted devices [35].
Q2: Which biosensor design generation is least susceptible to acetaminophen interference? A2: First-generation biosensor designs, which use oxygen as a cofactor, are typically more susceptible to acetaminophen interference. Manufacturers like Dexcom and Medtronic incorporate specialized interference and bioprotective membranes (domains) in their first-generation systems to mitigate this [4]. Second-generation designs that use a synthetic mediator can also be affected, though the specific interference profile depends on the mediator's redox potential and the presence of other mitigating membranes.
Q3: What experimental factors should I optimize to improve biosensor sensitivity and selectivity concurrently? A3: Key factors to optimize include:
- Electrode Geometry: Increasing the counter-to-working electrode area ratio (e.g., from 1:1 to 1:3) can enhance sensitivity [16].
- Functionalization Protocol: The method of enzyme immobilization (e.g., cross-linking with glutaraldehyde and BSA) impacts stability and activity [16].
- Coating Application: Using techniques like dip coating or electrochemical deposition to apply uniform, defect-free biocompatible layers is crucial for consistent interference rejection [35] [16].
Q4: My sensor signal is drifting. Is this related to the immobilization technique or coating failure? A4: Signal drift can be caused by both. Unstable enzyme immobilization can lead to leaching of glucose oxidase (GOx), causing a signal drop. Conversely, failure of the biocompatible coating, such as cracking, delamination, or biofouling, can alter the diffusion of glucose and interferents, leading to unpredictable signal drift. Inspection and testing of each layer are required for diagnosis.
Troubleshooting Guides
Issue: High Acetaminophen Interference
Problem: Sensor readings are falsely elevated in the presence of therapeutic levels of acetaminophen.
Investigation & Resolution:
| Investigation Step | Observation | Likely Cause & Solution |
|---|---|---|
| Verify Coating Integrity | No physical barrier to interferents | Coating is absent, too thin, or improperly applied. Solution: Re-apply a uniform Nafion coat via dip-coating [16]. |
| Check Sensor Design | Low counter-to-working electrode ratio | Suboptimal electrode geometry limits sensor performance. Solution: Optimize the counter-to-working electrode area ratio to at least 1:3 to improve sensitivity and performance [16]. |
| Review Immobilization | Unstable enzyme activity | Enzyme leaching from the electrode surface. Solution: Ensure robust cross-linking of GOx using glutaraldehyde and BSA [16]. |
Issue: Low Sensor Sensitivity
Problem: The sensor shows a weak current response to changes in glucose concentration.
Investigation & Resolution:
| Investigation Step | Observation | Likely Cause & Solution |
|---|---|---|
| Check Electrode Geometry | Counter electrode area is too small | Limited electrochemical reaction efficiency. Solution: Increase the surface area of the counter electrode to improve current density [16]. |
| Inspect Enzyme Layer | Inconsistent enzyme activity | Inadequate enzyme immobilization or deactivation. Solution: Standardize the cross-linking protocol with glutaraldehyde and BSA to ensure high enzyme loading and activity [16]. |
| Test Diffusion Layers | High diffusion barrier | The bioprotective membrane is too thick. Solution: Optimize the thickness of the diffusion-resistance membrane to balance glucose flux and linear range [4]. |
Protocol 1: Glucose Oxidase Immobilization via Cross-Linking
This protocol details the covalent immobilization of Glucose Oxidase (GOx) onto a Pt electrode surface for a stable and sensitive biosensor [16].
- Surface Preparation: Clean the Pt working electrode according to standard electrochemical procedures (e.g., polishing, cycling in acid).
- Enzyme Solution Preparation: Prepare a solution containing:
- 250,000 units/L Glucose Oxidase (from Aspergillus niger)
- 1% (w/v) Bovine Serum Albumin (BSA) in a suitable buffer (e.g., phosphate buffer saline).
- Cross-linking: Add glutaraldehyde to the enzyme-BSA solution to a final concentration of 0.1% (v/v). Mix gently.
- Immobilization: Deposit a small, precise volume (e.g., 5 µL) of the mixture onto the active area of the working electrode.
- Curing: Allow the electrode to dry at room temperature or at 4°C for 1-2 hours to complete the cross-linking process, forming an insoluble enzyme layer.
Protocol 2: Application of a Nafion Interference-Rejection Layer
This protocol describes dip-coating a sensor with Nafion to reduce the permeability of acetaminophen and other anionic interferents [16].
- Solution Preparation: Prepare a 0.5% - 2% (w/v) solution of Nafion in a suitable solvent (e.g., a lower aliphatic alcohol/water mixture).
- Coating Process: Dip the sensor with the immobilized enzyme layer into the Nafion solution.
- Withdrawal Control: Withdraw the sensor at a controlled, slow speed (e.g., 1-2 mm/sec) to ensure a uniform coating.
- Drying: Air-dry the sensor thoroughly for several hours to evaporate the solvent and form a stable, thin film.
Quantitative Data on Interference and Coating Efficacy
The following table summarizes key experimental findings on interference levels and the performance of mitigation strategies.
Table 1: Quantified Interference from Common Substances and Sensor Optimization Results [16]
| Parameter | Result / Value | Experimental Context |
|---|---|---|
| Acetaminophen Interference | Up to 150% | Measured at 5 mM glucose concentration without a Nafion coating. |
| Ascorbic Acid Interference | 4.5% - 17.8% | Measured at 5 mM glucose concentration without a Nafion coating. |
| Urea Interference | 4.2% - 11.3% | Measured at 5 mM glucose concentration without a Nafion coating. |
| Sensitivity Improvement | 0.63 to 1.28 µA/mm²·mM | Achieved by increasing the counter-to-working electrode area ratio from 1:1 to 1:3. |
| Limit of Detection (LoD) | 0.41 mM | Achieved with the optimized 1:3 electrode ratio. |
| Limit of Quantification (LoQ) | 1.12 mM | Achieved with the optimized 1:3 electrode ratio. |
Research Reagent Solutions
Table 2: Essential Materials for Biosensor Fabrication
| Reagent / Material | Function in Fabrication Protocol |
|---|---|
| Glucose Oxidase (GOx) | The biological recognition element that catalyzes the oxidation of glucose, producing the measurable signal [16]. |
| Glutaraldehyde | A cross-linking agent that creates covalent bonds between enzyme molecules and the BSA matrix, immobilizing them on the electrode surface [16]. |
| Bovine Serum Albumin (BSA) | Used as a stabilizing protein in the enzyme cocktail; it co-cross-links with GOx to form a robust, non-leaching biocomposite layer [16]. |
| Nafion | A perfluorinated ionomer used as a biocompatible coating to repel negatively charged interfering substances like acetaminophen and ascorbic acid [16]. |
| Hydroxyapatite | A bioceramic coating used primarily on orthopedic and dental implants to enhance osseointegration and bone bonding [35]. |
| Phosphorylcholine (PC) | A polymer coating that mimics the outer surface of cell membranes, providing high biocompatibility and reducing thrombogenicity and protein adsorption on blood-contacting devices [35]. |
Visualization of Concepts and Workflows
Biosensor Fabrication and Interference Mechanism
Troubleshooting and Optimization: Enhancing Sensor Robustness and Accuracy
Analyzing Commercial Sensor Failure Modes and Signal Drift
Troubleshooting Guides
FAQ: Understanding and Mitigating Sensor Drift
What is sensor drift and how can I identify it in my data? Sensor drift is a slow, progressive change in a sensor's output signal that occurs without any corresponding change in the actual measured quantity [36] [37]. Unlike random noise, which causes short-term fluctuations, drift is a consistent deviation that accumulates over months or years [37]. In your data, look for a gradual, consistent shift in baseline readings over time, even when measuring a stable reference. For example, a temperature sensor might read slightly warmer over time, or a pressure transducer may report consistently lower values despite steady pressure [38] [37].
What are the primary causes of drift in implantable biosensors? Drift in implantable biosensors stems from multiple factors:
- Aging Components: Electronic components like resistors and capacitors degrade over time, altering their electrical characteristics and stability [38] [37].
- Biological Fouling: Protein adsorption or cell growth on the sensor membrane can physically block analyte access and alter sensor response [39].
- Chemical Degradation: The sensing elements or membranes themselves can undergo irreversible chemical changes, especially when exposed to harsh bodily fluids [38] [40].
- Temperature Fluctuations: Changes in local temperature can cause materials within the sensor to expand or contract, altering its internal structure and calibration [36].
Which biosensor designs are most susceptible to acetaminophen interference, and why? First-generation electrochemical biosensors, which use oxygen as a natural electron acceptor, are particularly susceptible to acetaminophen interference [4]. These sensors, like certain models from Dexcom and Medtronic, operate at a high voltage to oxidize the hydrogen peroxide produced during the glucose oxidase reaction. At this high potential, easily oxidized substances like acetaminophen are also electrochemically active, generating a false current that is misinterpreted as higher glucose levels [4] [8]. Second-generation biosensors that use artificial mediators can operate at lower potentials and are generally less prone to this interference [4].
FAQ: Addressing Acetaminophen Interference
How does acetaminophen specifically interfere with continuous glucose monitors (CGMs)? Acetaminophen interferes because it is an electroactive species. In first-generation electrochemical CGMs, the sensor measures the current generated from the oxidation of hydrogen peroxide produced during the glucose oxidase reaction [8]. At the high voltage required for this reaction (~0.6-0.7 V), acetaminophen is also readily oxidized on the platinum working electrode [4] [41]. The sensor cannot distinguish between the current generated by hydrogen peroxide and that generated by acetaminophen, leading to a falsely elevated glucose reading [8]. The table below summarizes documented interference levels for specific CGM models.
Table 1: Documented Acetaminophen Interference in Commercial CGMs
| CGM Model (Manufacturer) | Biosensor Generation | Reported Interference Effect |
|---|---|---|
| Dexcom G6/G7 [4] | First | Taking >1000 mg every 6 hours may increase sensor readings. |
| Medtronic Guardian Connect (Sensor 4) [4] | First | May falsely raise sensor glucose readings; level of inaccuracy varies. |
| Medtronic Simplera [4] | First | May falsely raise sensor glucose readings. |
What design strategies can mitigate acetaminophen interference? Manufacturers employ several membrane strategies to reduce the flux of interferents like acetaminophen to the working electrode [4]:
- Permselective Membranes: Newer CGM models incorporate specialized membranes designed to be selectively permeable, allowing glucose to pass while hindering larger or differently charged molecules like acetaminophen [4].
- Nafion Composite Membranes: The negatively charged Nafion membrane can repel neutral species or selectively filter molecules based on charge, significantly reducing acetaminophen interference without affecting glucose sensitivity [41].
- Multi-Layer "Domain" Architecture: Advanced sensors use a stack of functional membranes, including an interference domain and a bioprotective domain, which work in concert to screen out common interferents [4].
What is the quantitative impact of intravenous acetaminophen on CGM readings? A 2025 case study on a patient using a Medtronic Guardian 4 sensor demonstrated a significant and rapid impact. After intravenous administration of 15 mg/kg acetaminophen, the CGM readings peaked at a mean of 29.2 ± 1.9 minutes after administration. The estimated discrepancy between the CGM reading and actual blood glucose ranged from 55 to 114 mg/dL [8]. The study also found a significant negative correlation, meaning the interference effect was greater at lower actual blood glucose levels, increasing the risk of hypoglycemia if an automated insulin delivery system reacts to the false high reading [8].
Table 2: Quantitative Impact of Intravenous Acetaminophen (15 mg/kg) on a Guardian 4 CGM [8]
| Metric | Result |
|---|---|
| Time to Peak CGM Discrepancy | 29.2 ± 1.9 minutes |
| Range of CGM Discrepancy | 55 to 114 mg/dL |
| Correlation with Blood Glucose | Significant negative correlation (greater discrepancy at lower glucose levels) |
Experimental Protocols
Protocol: In Vitro Characterization of Acetaminophen Interference
Objective: To quantitatively determine the cross-sensitivity of an implantable glucose biosensor to acetaminophen.
Materials:
- Phosphate Buffered Saline (PBS), pH 7.4
- D-Glucose stock solution
- Acetaminophen stock solution
- Potentiostat and electrochemical cell
- Biosensor(s) under test
Methodology:
- Calibration: Calibrate the biosensor in PBS with successive glucose additions (e.g., 0, 100, 200, 400 mg/dL). Record the steady-state current at each concentration to establish baseline glucose sensitivity.
- Interference Test:
- Return the solution to a baseline glucose level (e.g., 100 mg/dL).
- Add acetaminophen to achieve a clinically relevant concentration (e.g., 1.0 mg/dL, representing a therapeutic dose).
- Measure the steady-state sensor current.
- The observed increase in current is the direct interference signal from acetaminophen.
- Calculation: Calculate the apparent glucose error using the following formula. ( \text{Glucose Error (mg/dL)} = \frac{(I{AC} - I{Baseline})}{S} ) Where: ( I{AC} ) = Current with acetaminophen, ( I{Baseline} ) = Baseline current with glucose alone, ( S ) = Sensor sensitivity (nA/(mg/dL)) from Step 1.
Protocol: Evaluating Nafion Membranes for Interference Reduction
Objective: To assess the efficacy of a Nafion-coated membrane in reducing acetaminophen interference.
Materials:
- Biosensors with and without a Nafion composite membrane
- All materials listed in the previous protocol
Methodology:
- Grouping: Divide sensors into two groups: a test group with the Nafion membrane and a control group with a standard membrane.
- Glucose Sensitivity: Calibrate all sensors as in the previous protocol. Ensure both groups have statistically identical sensitivity to glucose.
- Acetaminophen Challenge: Expose all sensors to a solution containing a fixed glucose concentration and a high concentration of acetaminophen.
- Measurement: Record the sensor output and calculate the apparent glucose error for both groups.
- Analysis: Compare the mean glucose error between the Nafion-coated sensors and the control sensors. A successful coating will show a statistically significant reduction in the error. This method was validated in both rat and human studies [41].
Signaling Pathways and Workflows
Biosensor Interference Mechanism
Drift Analysis Workflow
The Scientist's Toolkit
Table 3: Essential Reagents and Materials for Interference & Drift Research
| Item | Function/Application | Key Detail / Rationale |
|---|---|---|
| Nafion Membrane | Selective barrier to reduce acetaminophen flux. | Negatively charged polymer that can repel or filter interferents; proven to significantly reduce acetaminophen signal in vivo [41]. |
| Phosphate Buffered Saline (PBS) | In vitro testing medium. | Provides a stable, physiologically relevant ionic background for baseline sensor characterization. |
| Acetaminophen (Standard) | Primary interferent for challenge studies. | Use high-purity (>98%) to prepare stock solutions for quantitative interference testing. |
| D-Glucose | Target analyte for calibration. | Used to establish baseline sensor sensitivity before interference testing. |
| Potentiostat | Core measurement instrument. | Applies potential to the sensor's working electrode and measures the resulting current with high precision. |
| Metal Oxide Sensor Arrays | For studying long-term drift patterns. | Useful for generating robust datasets on drift behavior over time, as in electronic nose research [40]. |
| Antibacterial agent 99 | Antibacterial Agent 99 | Antibacterial Agent 99 is a high-purity (99%) research-use-only (RUO) compound for in vitro studies. It inhibits essential bacterial processes. Not for human or veterinary use. |
| Anti-inflammatory agent 18 | Anti-inflammatory agent 18, MF:C30H50O6, MW:506.7 g/mol | Chemical Reagent |
Optimizing Electrode Configuration and Area Ratios for Improved Performance
For researchers developing implantable biosensors, mitigating the effects of interfering substances like acetaminophen is a significant challenge. Electrode configuration and area ratios present a powerful lever for enhancing sensor performance, directly impacting sensitivity, limit of detection, and interference resilience. This guide provides targeted troubleshooting and experimental protocols to address these key design parameters.
Frequently Asked Questions (FAQs)
FAQ 1: How does the counter-to-working electrode area ratio influence biosensor performance?
Optimizing the surface area ratio between the counter (CE) and working electrode (WE) is a critical step for enhancing signal response.
- Mechanism: A larger CE area improves the efficiency of the electrochemical cell by ensuring that the counter reaction is not a limiting factor. This reduces charge transfer resistance and increases the current density for the glucose oxidation reaction at the working electrode [16].
- Experimental Evidence: A study on microneedle-based biosensors demonstrated that increasing the CE:WE area ratio from 1:1 to 1:3 resulted in a dramatic 102% increase in sensitivity, from 0.63 µA/mm²·mM to 1.28 µA/mm²·mM [16]. This optimization also improved the Limit of Detection (LoD) to 0.41 mM [16].
FAQ 2: Which common substances interfere with electrochemical biosensors, and how can this be mitigated?
Interferents that oxidize at similar potentials to your target analyte can cause false positive signals. For implantable glucose biosensors, key interferents have been identified.
- Common Interferents:
- Acetaminophen: Causes significant positive interference in many first-generation biosensor designs [4] [16].
- Ascorbic Acid (Vitamin C): A known interferent for many CGM systems [4] [22].
- Hydroxyurea: Specifically listed as an interferent for Dexcom and Medtronic CGM systems [4].
- Urea: Typically shows minimal interference [16].
- Mitigation Strategies:
- Permselective Membranes: Coating the electrode with a Nafion membrane can significantly improve specificity by reducing the flux of interfering substances to the electrode surface [4] [16].
- Sensor Design Evolution: Manufacturers continuously update designs; for example, the Dexcom G6/G7 and FreeStyle Libre 2 Plus/3 Plus incorporate features to reduce acetaminophen and ascorbic acid interference, respectively [4].
FAQ 3: What are the key material and design considerations for a stable working electrode?
The choice of materials and fabrication methods directly affects electrode stability, conductivity, and active surface area.
- Material Selection:
- Carbon-Based Materials: Promising for low-cost, disposable sensors. A hybrid electrode of graphite flakes/carbon fiber (G/CF) creates a "3D highway network" for efficient electron transfer, offering high conductivity and a large active surface area [42].
- Conductive Fibers: Low-cost steel fibers can serve as a robust base for working electrodes, which can be modified with materials like gold-nanoparticle-doped carbon ink to enhance their electrochemical properties [43].
- Functionalization: Enzymes like Glucose Oxidase (GOx) are often immobilized using cross-linking agents (e.g., glutaraldehyde) and stabilizers (e.g., Bovine Serum Albumin) to maintain activity and stability [16].
Troubleshooting Guides
Problem: Low Sensor Sensitivity or Poor Signal Response
| Possible Cause | Diagnostic Steps | Recommended Solution |
|---|---|---|
| Suboptimal CE:WE Area Ratio | 1. Calculate the current surface area of your CE and WE.2. Perform cyclic voltammetry in a standard solution to assess current output. | Increase the counter electrode area relative to the working electrode. Test ratios of 1:2 and 1:3 (CE:WE) to maximize sensitivity [16]. |
| Insufficient Enzyme Loading or Activity | 1. Verify enzyme concentration and immobilization protocol.2. Test sensor response in a standard glucose solution. | Optimize enzyme loading and use a cross-linker like PEGDGE. Employ response surface methodology (e.g., Box-Behnken design) to find the ideal enzyme/cross-linker balance [44]. |
| High Impedance at Electrode Interface | Perform Electrochemical Impedance Spectroscopy (EIS) to measure charge transfer resistance. | Use electrode materials with higher conductivity (e.g., platinum, gold) or nanostructured materials like the G/CF hybrid to enhance surface area and electron transfer [42]. |
Problem: High Signal Interference from Acetaminophen
| Possible Cause | Diagnostic Steps | Recommended Solution |
|---|---|---|
| Lack of a Selective Membrane | Test sensor response in a solution containing only the interferent (e.g., acetaminophen in buffer). A significant signal indicates poor selectivity. | Apply a permselective Nafion coating to the working electrode. This polymer layer can block negatively charged interferents like ascorbate and urate while allowing Hâ‚‚Oâ‚‚ (the signal molecule) to pass [16]. |
| Incorrect Operating Potential | The applied potential may be high enough to directly oxidize the interferent. | If possible, lower the operating potential of the sensor. Second-generation biosensors use mediators to achieve this, reducing the impact of interferents [4]. |
| Saturation from High Acetaminophen Doses | Review the clinical context; therapeutic doses can exceed 1 g [22]. | Acknowledge this limitation in your research. For in vivo applications, refer to manufacturer guidelines, which often warn of interference at high doses (e.g., >1000 mg per 6 hours for Dexcom G6/G7) [4]. |
Experimental Data & Protocols
Quantified Impact of Electrode Ratios and Interferents
The table below summarizes key quantitative findings from recent studies to guide your experimental design and expectations.
Table 1: Performance Metrics from Biosensor Optimization Studies
| Optimization Parameter | Baseline Performance | Optimized Performance | Key Finding / Context |
|---|---|---|---|
| CE:WE Area Ratio [16] | 1:1 Ratio | 1:3 Ratio | |
| Sensitivity (µA/mm²·mM) | 0.63 | 1.28 | 102% sensitivity increase. |
| Limit of Detection (mM) | Not Specified | 0.41 | Improved LoD with larger ratio. |
| Chemical Interference [16] | Tested at 5 mM Glucose | ||
| Acetaminophen Interference | --- | Up to 150% | Highest interference effect. |
| Ascorbic Acid Interference | --- | 4.5 - 17.8% | Minimal to moderate interference. |
| Urea Interference | --- | 4.2 - 11.3% | Minimal interference. |
| Lactate Sensor Optimization [44] | Box-Behnken Design Used | ||
| Oxidation Current (µA) | Various | 1840 ± 60 | With 4 layers of LOx (1.9 U) & PEGDGE (184 µg). |
Detailed Protocol: Optimizing Electrode Area Ratio
This protocol provides a methodology for systematically evaluating and optimizing the counter-to-working electrode area ratio.
Procedure:
- Design & Fabrication: Fabricate multiple electrode designs where the counter electrode surface area is varied relative to the working electrode. Common ratios to test are 1:1, 1:2, and 1:3 (CE:WE) [16].
- Functionalization: Immobilize your biorecognition element (e.g., Glucose Oxidase) onto the working electrode using a standardized protocol involving cross-linkers like glutaraldehyde and BSA [16]. Apply a Nafion coating if interference is a concern.
- Electrochemical Testing: Perform amperometric or voltammetric measurements in a series of standard glucose solutions (e.g., 0-30 mM) for each electrode design.
- Data Analysis:
- Plot the steady-state current against glucose concentration for each ratio.
- Calculate the sensitivity (slope of the calibration curve).
- Calculate the Limit of Detection (LoD) using the formula 3.3 × σ/S, where σ is the standard deviation of the blank response and S is the sensitivity of the calibration curve [16].
- Selection: Identify the electrode ratio that yields the highest sensitivity and lowest LoD for further development.
Detailed Protocol: Testing for Acetaminophen Interference
This protocol assesses the impact of acetaminophen on your biosensor's accuracy.
Procedure:
- Solution Preparation:
- Solution A (Control): A buffer solution containing a known, fixed concentration of glucose (e.g., 5 mM).
- Solution B (Interference Test): The same buffer with the identical concentration of glucose, plus a physiologically relevant concentration of acetaminophen (e.g., 0.2 mM or higher, based on the therapeutic range you are targeting) [43] [45].
- Measurement: Record the stable amperometric signal of your biosensor first in Solution A and then in Solution B.
- Calculation: Quantify the percentage interference using the formula:
- % Interference = (Signal in Solution B - Signal in Solution A) / Signal in Solution A × 100%
- Refer to Table 1 for benchmark values from recent research [16].
- Interpretation: If the interference exceeds your acceptable threshold (e.g., >10%), implement mitigation strategies such as applying a Nafion coating or re-optimizing the operating potential.
The Scientist's Toolkit
Table 2: Essential Research Reagents and Materials
| Item | Function / Role in Development | Example Context |
|---|---|---|
| Nafion | A permselective polymer coating used to block anionic interferents (e.g., ascorbate, urate) and improve sensor selectivity [16]. | Applied over the glucose oxidase layer on a Pt-working electrode to reduce acetaminophen interference [16]. |
| Glutaraldehyde & BSA | Cross-linking agents used to covalently immobilize enzymes (e.g., GOx) onto the electrode surface, ensuring stability and retention of enzyme activity [16]. | Used in a mixture with GOx and BSA to functionalize a platinum microneedle working electrode [16]. |
| PEGDGE (Poly(ethylene glycol) diglycidyl ether) | A cross-linking agent for creating stable, biocompatible matrices for enzyme immobilization. Optimizing its loading is crucial for sensitivity and stability [44]. | Used with Lactate Oxidase (LOx) on a carbon paper electrode to create a highly stable and sensitive lactate biosensor [44]. |
| Graphite/Carbon Fiber (G/CF) Hybrid | A composite electrode material that creates a 3D conductive network, offering high surface area, low impedance, and enhanced electron transfer compared to standard carbon electrodes [42]. | Used as the base for a working electrode to achieve superior sensitivity for glucose detection without nanomaterials [42]. |
| Conductive Steel Fiber | A low-cost, mechanically robust conductive yarn that can be embroidered or integrated into flexible electrochemical sensors as a base electrode material [43]. | Served as the working electrode foundation for a textile-based sensor to detect acetaminophen in breast milk [43]. |
| D-Sorbitol-13C-2 | D-Sorbitol-13C-2, MF:C6H14O6, MW:183.16 g/mol | Chemical Reagent |
Protocols for Dynamic In Vitro Interference Testing and Validation
Accurate measurement of biomarkers is fundamental to the performance of implantable biosensors. A significant challenge in this field is the distortion of sensor signals caused by interfering substances that are common in a physiological environment. Acetaminophen, a widely used over-the-counter analgesic, is a classic example of such an interferent for continuous glucose monitoring (CGM) systems. Falsely elevated glucose readings can trigger inappropriate therapeutic decisions, such as incorrect insulin dosing, posing a direct risk to patient safety [13] [8]. This technical support center provides detailed protocols, troubleshooting guides, and FAQs to support researchers in developing robust dynamic in vitro interference tests, a critical step in validating and improving the reliability of implantable biosensors.
Core Experimental Protocol
Dynamic Interference Testing Methodology
Traditional static interference tests are insufficient for simulating the constantly changing in vivo conditions. The following protocol, adapted from Pfützner et al. (2024), outlines a method for dynamic interference testing that can reproduce more physiologically relevant conditions [46].
Principle: A macrofluidic test stand uses programmable high-pressure liquid chromatography (HPLC) pumps to generate dynamic concentration gradients of both glucose and potential interferents, such as acetaminophen, in a controlled solution. The sensor's response is tracked in real-time and compared to a reference method [46].
Materials and Equipment:
- Macrofluidic Test Stand: Custom-designed flow system.
- HPLC Pumps: For generating precise, programmable gradients.
- Sensor Holders: To securely house the implantable biosensor(s) under test (e.g., CGM needle sensors).
- Reference Glucose Analyzer: e.g., YSI 2300 Stat Plus.
- Test Solution: Phosphate-buffered saline (PBS) as the base matrix.
- Analytes: Glucose and interfering substances (e.g., acetaminophen, maltose, xylose).
- Data Acquisition System: To record sensor and reference data.
Procedure:
- System Setup: Calibrate the HPLC pumps and reference analyzer. Place the biosensor in the test chamber and ensure all fluidic connections are secure.
- Baseline Establishment: Flow pure PBS buffer through the system to establish a stable sensor baseline.
- Dynamic Glucose Challenge: Program the pumps to generate a dynamic glucose profile in PBS. This tests the sensor's fundamental performance in tracking glucose changes without interferents.
- Interference Testing: Introduce a gradient of the interferent (e.g., acetaminophen). This can be done:
- With stable background glucose to isolate the interferent's effect.
- Simultaneously with a glucose gradient to assess interference under more complex, realistic conditions.
- Data Collection: Continuously record the sensor signal and simultaneously take measurements with the reference analyzer at predefined intervals.
- Data Analysis: Calculate performance metrics such as Mean Absolute Relative Difference (MARD) and analyze the sensor's deviation from the reference in the presence of the interferent.
Figure 1: Experimental workflow for dynamic in vitro interference testing.
Key Research Reagent Solutions
The table below catalogs essential materials and their functions for establishing a dynamic interference testing platform.
Table 1: Essential Reagents and Materials for Dynamic Interference Testing
| Item | Function/Description | Example from Literature |
|---|---|---|
| Macrofluidic Test Stand | Generates programmable, dynamic concentration gradients of glucose and interferents in a controlled fluidic environment [46]. | Custom-built system with HPLC pumps [46]. |
| Phosphate-Buffered Saline (PBS) | Provides a stable, buffered saline solution that mimics physiological ionic strength and pH, serving as the base matrix for tests [46]. | Used as the fluidic environment in validated protocols [46]. |
| Reference Glucose Analyzer | Serves as the gold-standard reference method against which the sensor's performance is validated (e.g., YSI 2300 Stat Plus) [46]. | YSI 2300 Stat Plus [46]. |
| Acetaminophen (Paracetamol) | A well-characterized electrochemical interferent for amperometric glucose sensors; used as a positive control in interference tests [13] [8]. | Used in both in vitro [46] and clinical studies [13] [8]. |
| Maltose & Xylose | Substances used as negative or positive controls depending on the sensor technology. Maltose does not affect some CGM systems, while xylose can interfere with others [46]. | Used for validation in dynamic test setups [46]. |
Troubleshooting Guides and FAQs
Frequently Asked Questions
Q1: Why is dynamic testing preferred over static testing for interference validation?
Static tests, which use constant concentrations, cannot replicate the changing conditions of the human body. Dynamic testing with concentration gradients reveals how a sensor responds to the rising and falling levels of an interferent, providing a more realistic and rigorous assessment of its performance and potential for signal drift in vivo [46].
Q2: Our sensor shows significant signal drift during long-term testing. What could be the cause?
Baseline drift can originate from multiple sources. In a flow system, inefficient regeneration of the sensor surface between runs can cause a buildup of material. It is also critical to ensure buffer compatibility, as certain components can destabilize the sensor surface. Finally, always verify that the instrument itself is properly calibrated [47].
Q3: What are the clinical implications of acetaminophen interference for automated insulin delivery systems?
Falsely elevated CGM readings due to acetaminophen can be dangerous. In an Automated Insulin Delivery (AID) system, these false highs could trigger an unneeded "autocorrection" insulin bolus, potentially leading to a dangerous hypoglycemic event. This risk is heightened at lower actual glucose levels, where the relative discrepancy is greater [8].
Q4: Are there regulatory considerations for validating in-house developed tests (LDTs) for sensor validation?
Yes. In the European Union, the In Vitro Diagnostic Regulation (IVDR) applies. Laboratories developing in-house tests must operate under a quality management system (e.g., ISO 15189), ensure their devices meet general safety and performance requirements (Annex I GSPRs), and justify their use over commercially available CE-marked tests [48].
Troubleshooting Common Experimental Issues
Problem: Non-specific binding causing high background signal.
- Potential Causes: Sample impurities, suboptimal surface chemistry, or inadequate blocking.
- Solutions:
- Improve sample purification to remove aggregates and contaminants.
- Optimize surface blocking using agents like BSA or casein.
- Incorporate detergent additives (e.g., Tween-20) in the running buffer to minimize non-specific adsorption [47].
Problem: Poor reproducibility between experimental runs.
- Potential Causes: Inconsistent sensor surface preparation, ligand immobilization, or environmental fluctuations.
- Solutions:
- Standardize all surface activation and ligand coupling protocols with careful control of time, temperature, and pH.
- Always include negative controls to monitor for non-specific binding and system errors.
- Perform experiments in a temperature-controlled environment to minimize external variability [47].
Problem: Low signal-to-noise ratio.
- Potential Causes: Insufficient ligand density on the sensor surface, low immobilization efficiency, or a weak interaction.
- Solutions:
- Titrate the ligand during immobilization to find the optimal surface density that balances signal strength and avoids steric hindrance.
- Use sensor chips with enhanced sensitivity for detecting weak interactions or low-abundance analytes.
- If the interaction is weak, a moderate increase in analyte concentration may be necessary, avoiding levels that cause saturation [47].
Data Presentation and Analysis
Quantifying the interference effect is crucial for reporting and comparison. The following table summarizes key quantitative findings from clinical and laboratory studies on acetaminophen interference.
Table 2: Quantified Interference Effects of Acetaminophen on CGM Systems
| Sensor / System | Acetaminophen Dose & Route | Observed Effect | Key Quantitative Findings |
|---|---|---|---|
| Dexcom G4 PLATINUM [13] | 1000 mg, Oral | Falsely elevated CGM readings | Max mean difference: 61 mg/dL at 120 min (BG meter: 171 ± 37 mg/dL). Effect lasted up to 8 hours. |
| Guardian 4 [8] | 15 mg/kg, IV (15 min infusion) | Rapid, sharp increase in CGM readings | Peak CGM at 29.2 ± 1.9 min. Estimated discrepancy: 55-114 mg/dL. Greater effect at lower glucose levels. |
| Dexcom G6 & FreeStyle Libre 2 [46] | Gradient, In Vitro | Confirmed susceptibility | G6 readings susceptible to acetaminophen. L2 readings susceptible to xylose. Validated dynamic test protocol. |
Regulatory and Clinical Context
The transition towards more automated and closed-loop therapy systems, like AID, makes mitigating interference an engineering and regulatory imperative. Sensor accuracy is not just a performance metric but a critical patient safety feature [49] [8]. Regulatory frameworks like the EU's IVDR emphasize the need for a robust, transparent demonstration of device safety and performance, which directly applies to the thorough validation of interference effects [48]. The dynamic in vitro protocols described here provide a rigorous methodology to generate the high-quality data required for regulatory submissions and, ultimately, for building safer medical devices.
Post-Processing Algorithms for Compensating Acetaminophen-Induced Signal Bias
Frequently Asked Questions (FAQs)
Q1: What is the fundamental cause of acetaminophen interference in electrochemical biosensors? Acetaminophen (APAP) is an electroactive compound that oxidizes at a potential close to that of hydrogen peroxide (Hâ‚‚Oâ‚‚), which is the primary product detected by most glucose oxidase-based enzyme sensors. When the sensor's working electrode measures the current generated by Hâ‚‚Oâ‚‚ oxidation, the simultaneous oxidation of acetaminophen contributes an additional, non-glucose-related current. This results in a falsely elevated glucose reading [50] [14].
Q2: Which continuous glucose monitoring (CGM) systems are known to be affected by acetaminophen interference? Independent in vitro testing has revealed that different CGM systems exhibit varying susceptibilities. The Dexcom G6 sensor has been shown to be highly susceptible to acetaminophen interference, producing significant positive signal bias. In contrast, the Abbott Libre 2 sensor, when tested against the same panel of substances, did not show significant interference from acetaminophen [19].
Q3: What are the primary strategies for mitigating acetaminophen interference? Two main approaches exist:
- Physical Barrier Membranes: Using a composite Nafion membrane on the sensor can selectively reduce the diffusion of acetaminophen to the electrode surface, thereby diminishing its interference. This has been demonstrated in both rodent and human studies [50].
- Post-Processing Algorithms: Software-based corrections can be applied to the raw sensor signal. These algorithms can be designed to recognize the distinctive signature of acetaminophen interference (e.g., its specific oxidation potential or temporal profile) and subtract the estimated interfering current to report a corrected glucose value.
Q4: How can I experimentally validate the performance of a compensation algorithm in a laboratory setting? A robust method involves using a dynamic in vitro test bench. This setup exposes sensors to a stable glucose background (e.g., 200 mg/dL) while introducing a linearly increasing and then decreasing concentration of acetaminophen. The sensor's output is compared against a reference method (like a YSI analyzer) throughout the experiment. A successful algorithm will minimize the bias between the sensor reading and the reference value across all acetaminophen concentrations [19].
Q5: Beyond pharmaceuticals, why is detecting acetaminophen in environmental samples important? Acetaminophen is considered an emerging environmental contaminant. It is frequently detected in surface and wastewater due to widespread use and incomplete removal in treatment plants. Its environmental transformation products, such as 1,4-benzoquinone, can be more toxic than the parent compound, necessitating sensitive monitoring techniques [51].
Troubleshooting Guides
Issue: Inconsistent Algorithm Performance Across Subjects
Problem: A post-processing algorithm that works well in vitro or in one animal model fails to consistently correct for acetaminophen interference when tested in a larger, more diverse population.
| Potential Cause | Diagnostic Steps | Recommended Solution |
|---|---|---|
| Variable Pharmacokinetics | Measure plasma and interstitial fluid (ISF) acetaminophen concentrations over time. Compare the temporal profile of ISF APAP with the sensor error. | Develop a population pharmacokinetic model to inform the algorithm. Incorporate individual covariates (e.g., weight, renal function) to personalize the correction. |
| Sensor Biofouling | Examine explanted sensors for protein adsorption and cellular buildup. Test algorithm performance on sensors before and after a simulated biofouling protocol. | Implement a time-dependent calibration factor in the algorithm that adapts to the changing sensor sensitivity throughout its functional lifetime. |
| Metabolite Interference | Use chromatographic methods (e.g., LC-MS) to identify and quantify APAP metabolites (e.g., APAP-glucuronide, APAP-sulfate) in the ISF. | Refine the algorithm to account for the electrochemical activity of major metabolites, not just the parent acetaminophen compound. |
Issue: Algorithm Introduces Delay in Glucose Reporting
Problem: The application of the compensation filter successfully reduces acetaminophen-induced bias but results in a clinically significant lag time in the reported glucose values, especially during periods of rapid glucose change.
| Potential Cause | Diagnostic Steps | Recommended Solution |
|---|---|---|
| Overly Aggressive Filtering | Analyze the phase response of the digital filter used in the algorithm. Test the algorithm on datasets with sharp glucose excursions. | Switch to a finite impulse response (FIR) filter or a forward-looking Kalman filter to minimize phase distortion while maintaining effective noise and interference rejection. |
| Incorrect Signal Modeling | Deconstruct the raw sensor signal into its hypothesized components: glucose, acetaminophen, baseline drift, and noise. | Use a state-space modeling approach where the glucose and interference states are estimated simultaneously, rather than applying a sequential correction, to improve real-time accuracy. |
Quantitative Data on Acetaminophen Interference
The following table summarizes key findings from critical studies on acetaminophen interference and sensor performance.
Table 1: Summary of Experimental Findings on Acetaminophen Interference
| Study Context | Sensor / Electrode Type | Key Quantitative Findings | Reference Method |
|---|---|---|---|
| In Vivo (Human) CGM Interference | Dexcom G4 Platinum, Medtronic Guardian | Oral 1g APAP (Plasma ~140 μmol/L) caused CGM readings to spike from ~5 mM (90 mg/dL) to ~22 mM (400 mg/dL) while plasma glucose remained constant. ISF APAP concentration correlated with interference timing [14]. | YSI Analyzer, Microdialysis + COBAS c311 |
| In Vitro CGM Screening | Dexcom G6 (G6), Abbott Libre 2 (L2) | At a stable glucose of 200 mg/dL, APAP caused a >+100% bias in G6 readings. No significant interference was observed with the L2 sensor [19]. | YSI Stat 2300 Plus |
| Membrane Mitigation (Rat & Human) | Nafion-coated vs. Non-Nafion Glucose Sensors | Nafion coating reduced in vitro sensitivity to APAP from 30.8 to 12.2 nA·mmolâ»Â¹Â·Lâ»Â¹. In vivo, the current generated by APAP infusion was reduced from 2.0 nA (non-Nafion) to 0.5 nA (Nafion) [50]. | Not Specified |
| Electrochemical Detection | α-Bi₂O₃ Modified Electrode | Linear detection range: 0.05 - 12.00 μM. Limit of Detection (LOD): 10 nM. Successfully applied to pharmaceutical formulation analysis [52]. | Differential Pulse Voltammetry |
Table 2: Performance of Selected Electrochemical Sensors for Acetaminophen Detection
| Sensor Modifier | Electrode Base | Linear Range (μmol Lâ»Â¹) | Limit of Detection (LOD) | Sample Matrix | Ref. |
|---|---|---|---|---|---|
| Reduced Graphene Oxide (RGO) | Glassy Carbon Paste | 1.2 - 220 | 0.31 μmol Lâ»Â¹ | Human Urine, Tablets | [53] |
| Gold-Nanoparticle Carbon Ink | Textile (Steel Yarn) | 9.9 - 166.4 | 1.15 μmol Lâ»Â¹ | Human Breast Milk | [43] |
| Rod-shaped α-Biâ‚‚O₃ | Glassy Carbon Paste | 0.05 - 12.00 | 0.01 μmol Lâ»Â¹ | Pharmaceuticals | [52] |
Experimental Protocols
Protocol: Dynamic In Vitro Interference Testing for CGM Sensors
This protocol, adapted from [19], provides a standardized method for assessing the impact of acetaminophen on sensor performance.
Objective: To dynamically evaluate the interference effect of acetaminophen on a continuous glucose monitor's signal at a constant glucose concentration.
Materials and Reagents:
- CGM sensors (e.g., Dexcom G6, Abbott Libre 2)
- HPLC pump or equivalent precision pump system
- Temperature-controlled chamber (maintained at 37°C)
- Phosphate-Buffered Saline (PBS), pH 7.2
- D-Glucose
- Acetaminophen (APAP) stock solution
- Reference glucose analyzer (e.g., YSI Stat 2300 Plus)
- 3D-printed or custom fluidic channel test bench
Procedure:
- Setup: Place the CGM sensors into the fluidic channel according to the manufacturer's instructions. Connect the channel to the pump system.
- Baseline Perfusion: Perfuse the channel with PBS solution containing a stable, high concentration of glucose (e.g., 200 mg/dL) at a constant flow rate (e.g., 1 mL/min). Allow the system to equilibrate for at least 30 minutes until a stable sensor baseline is established.
- Sample Collection: Begin collecting effluent from the channel outlet at regular intervals (e.g., every 10-15 minutes) for immediate analysis with the reference glucose analyzer.
- Interference Test: After the baseline period, initiate a second pump containing the APAP solution dissolved in the same glucose-PBS buffer. Program the pump to create a linear gradient, increasing the APAP concentration from 0% to 100% of the target maximum over 60 minutes.
- Sustain Phase: Maintain the APAP concentration at its maximum level for 30 minutes.
- Washout Phase: Program the pump to linearly decrease the APAP concentration back to 0% over 60 minutes, followed by a final 30-minute washout period with APAP-free glucose-PBS buffer.
- Data Analysis: Compare the CGM sensor readings with the reference glucose values throughout the experiment. Calculate the percent bias from baseline for each sensor. Interference is typically defined as a mean bias of ≥ ±10%.
Protocol: Voltammetric Detection of Acetaminophen using a Modified Electrode
This protocol outlines a general method for detecting and quantifying acetaminophen using an electroanalytical approach, as exemplified in [53] [52].
Objective: To quantify the concentration of acetaminophen in a solution using square-wave or differential pulse voltammetry.
Materials and Reagents:
- Potentiostat/Galvanostat
- Three-electrode system: Modified Working Electrode (e.g., RGO/GCPE, Bi₂O₃/GCP), Ag/AgCl Reference Electrode, Platinum Counter Electrode
- Acetate buffer (0.1 M, pH 5.0) or Britton-Robinson buffer
- Acetaminophen standard solutions (e.g., 1 mM stock in deionized water)
- Sample for analysis (e.g., diluted pharmaceutical, filtered urine)
Procedure:
- Electrode Preparation: Prepare the modified working electrode according to the specific literature protocol (e.g., hand-mixing carbon paste with modifier, packing into electrode body, and smoothing the surface).
- Instrument Setup: Place the electrodes in an electrochemical cell containing the supporting electrolyte (e.g., acetate buffer). Connect the electrodes to the potentiostat.
- Electrochemical Cleaning (Optional): Perform cyclic voltammetry in the clean supporting electrolyte between a suitable potential window (e.g., 0.0 to +1.0 V) for several cycles until a stable background is obtained.
- Standard Curve Acquisition:
- Record a voltammogram of the blank supporting electrolyte.
- Add known aliquots of the acetaminophen standard solution to the cell. After each addition and stirring, allow the solution to become quiescent.
- For each concentration, run the voltammetric method (e.g., Square-Wave Voltammetry: potential step 5 mV, amplitude 50 mV, frequency 40 Hz; scan from 0.0 to +1.0 V).
- Measure the oxidation peak current (or peak area) at approximately +0.5 V vs. Ag/AgCl.
- Sample Analysis: Introduce the prepared sample into the electrochemical cell. Record the voltammogram under identical conditions.
- Quantification: Construct a calibration curve by plotting the peak current (or area) against the concentration of the APAP standards. Use the linear regression equation from this curve to determine the concentration of APAP in the unknown sample.
Signaling Pathways and Workflows
Mechanism of Acetaminophen Interference in Glucose Oxidase Biosensors
The following diagram illustrates the simultaneous electrochemical reactions that lead to signal bias in glucose sensors.
Workflow for Developing a Compensation Algorithm
This diagram outlines a systematic research workflow for creating and validating a post-processing algorithm to correct for acetaminophen interference.
The Scientist's Toolkit: Key Research Reagents & Materials
Table 3: Essential Materials for Investigating Acetaminophen Interference
| Item | Function / Application | Example from Literature |
|---|---|---|
| Nafion Membrane | A perm-selective polymer coating that can be applied to sensor electrodes to reduce the diffusion of negatively charged interferents like acetaminophen and urate, while allowing Hâ‚‚Oâ‚‚ to pass. | Used in a composite membrane to successfully reduce APAP interference in implanted glucose sensors in rats and humans [50]. |
| Microdialysis System | A technique for sampling and quantifying analytes from the interstitial fluid (ISF). Critical for in-vivo validation, allowing direct measurement of ISF acetaminophen concentrations alongside sensor performance. | Used to confirm that the temporal profile of CGM interference directly follows ISF acetaminophen levels [14]. |
| Nanomaterial Modifiers (e.g., RGO, Bi₂O₃) | Substances used to modify working electrodes to enhance sensitivity, lower the overpotential, and improve selectivity for acetaminophen detection in electroanalytical methods. | RGO modified glassy carbon paste electrodes for urine/tablet analysis [53]; Rod-shaped α-Bi₂O₃ for highly sensitive detection in pharmaceuticals [52]. |
| Dynamic In Vitro Test Bench | A controlled system (fluidic channel, precision pumps, temperature control) for exposing sensors to dynamically changing concentrations of interferents at a stable glucose background. Enables high-throughput, reproducible interference screening. | Used to test 68 substances on Abbott L2 and Dexcom G6 sensors, identifying APAP as a major interferent for the G6 [19]. |
| Reference Analyzer (e.g., YSI) | A high-accuracy benchtop instrument used to provide the "true" glucose concentration in solution during in-vitro experiments. Serves as the gold standard against which sensor accuracy and algorithm performance are measured. | Used as the reference method in dynamic interference testing protocols [19]. |
Designing Sensors for Resilience in Complex Biofluids
Core Concepts: Understanding Sensor Interference
What is the primary cause of acetaminophen interference in electrochemical biosensors? Acetaminophen is an electrochemically active substance that can be oxidized at a sensor's working electrode, generating a current that is indistinguishable from the signal produced by the target analyte (e.g., glucose). This leads to falsely elevated readings [4].
How do different biosensor generations handle interferents like acetaminophen? Biosensor designs employ different strategies to mitigate interference, which can be categorized by generation:
- First-Generation (e.g., Dexcom G6/G7, Medtronic systems): Rely on oxygen as a natural electron shuttle. They often incorporate specialized permselective membranes designed to reduce the flux of interfering substances, like acetaminophen, to the electrode surface [4].
- Second-Generation (e.g., Abbott FreeStyle Libre systems): Use an artificial mediator to shuttle electrons. These systems are typically less susceptible to acetaminophen but may have other labeled interferents, such as ascorbic acid (Vitamin C) [4].
- Third-Generation (e.g., Sinocare iCan i3): Engineered for direct electron transfer from the enzyme to the electrode, potentially offering reduced interference from common substances, with some manufacturers claiming no acetaminophen or vitamin C interference [4].
What design features improve sensor resilience in complex biofluids? Key design approaches include:
- Interference-Reducing Membranes: Multi-layered membranes or "domains" act as physical and chemical barriers. These can include a bioprotective membrane to prevent biofouling and an interference membrane specifically tuned to limit the passage of substances like acetaminophen [4].
- Advanced Materials: Novel coatings, such as cross-linked protein nanocomposites with functionalized graphene, are being developed to prevent non-specific protein attachment (biofouling) and microbial growth, thereby maintaining sensor performance and longevity in complex environments like the interstitial fluid [54].
- Molecularly Imprinted Polymers (MIPs): These synthetic polymers create specific recognition sites for a target molecule, acting like a "lock and key." This technology can be harnessed to selectively capture and measure interferents like acetaminophen for accurate quantification, or potentially to block their access to the sensing element [55].
Troubleshooting Guide: FAQs on Acetaminophen Interference
FAQ 1: Our in-vivo sensor readings are consistently higher than reference values when testing with animal models. We suspect drug interference. What is the first step in diagnosing this? The first step is to conduct a systematic in-vitro interference screening. Prepare a controlled buffer solution (e.g., PBS, pH 7.4) containing a known concentration of your target analyte. Acquire a stable baseline sensor signal. Then, spike the solution with acetaminophen at clinically relevant concentrations (typically up to 1-2 mg/L in plasma, but can be higher post-dose). A significant signal shift confirms interference. Compare the magnitude of the signal change to the signal from your target analyte to quantify the interference effect [4] [55].
FAQ 2: We are developing a novel glucose sensor and need to validate its specificity against acetaminophen. What is a robust experimental protocol? A standard protocol involves amperometric measurement under controlled potential.
- Setup: Use a standard electrochemical cell (working electrode, counter electrode, reference electrode) with your sensor as the working electrode.
- Baseline: Immerse the sensor in a continuously stirred, deaerated buffer (e.g., 0.1 M PBS, pH 7.4) at 37°C. Apply the operating potential and record the baseline current until stable.
- Glucose Response: Add aliquots of a stock glucose solution to achieve increasing concentrations across the physiological range (e.g., 2-20 mM). Record the steady-state current at each concentration to establish a calibration curve.
- Interference Test: Return to the baseline buffer. Subsequently, add acetaminophen to achieve a high therapeutic concentration (e.g., 200-300 µM). Record the change in current.
- Analysis: Calculate the apparent glucose equivalent signal caused by acetaminophen. The interference is often reported as the positive bias in mg/dL or mM glucose caused by the interferent [4].
FAQ 3: Our sensor's anti-fouling coating is failing in complex biofluids like serum, leading to drift and reduced performance. What material strategies can we explore? Consider implementing a multi-functional nanocomposite coating. A promising approach involves a matrix of cross-linked bovine serum albumin (BSA), which is highly non-fouling, integrated with pentaamine-functionalized reduced graphene oxide for electrical conductivity and stability. Covalently binding broad-spectrum antibiotics into this matrix can further prevent microbial attachment and biofilm formation. This composite has been shown to be non-toxic to primary human cells while effectively resisting non-specific protein, microbial, and fibroblast attachment, which is critical for long-term implantable sensor stability [54].
FAQ 4: How can we achieve highly selective detection of acetaminophen itself for therapeutic drug monitoring applications? A highly effective method combines molecularly imprinted polymers (MIPs) with advanced 2D nanomaterials. The MIP provides selectivity by creating specific binding cavities for acetaminophen, while nanomaterials like MXenes (e.g., Ti3C2Tx) enhance signal transduction. Incorporating porous carbon derived from Zeolitic Imidazolate Frameworks (ZIF-8) can prevent the restacking of MXene nanosheets, increasing the active surface area and stability. This MIP/MXene/C-ZIF-8 composite on a glassy carbon electrode has demonstrated a very low detection limit (2.775 nM) and a wide linear range (10â»Â³ - 10â»â· M) for acetaminophen [55].
Table 1: Summary of Key Experimental Protocols for Addressing Sensor Interference
| Experiment Goal | Core Methodology | Key Parameters to Measure | Common Validation Techniques |
|---|---|---|---|
| In-vitro Interference Screening | Amperometric measurement in buffer with sequential spiking of target analyte and interferent (e.g., acetaminophen). | Signal change (current) versus concentration for both analyte and interferent. | Calculation of interference as glucose equivalent bias; comparison to manufacturer claims [4]. |
| Sensor Selectivity Validation | Exposing the sensor to a panel of potential interferents (e.g., acetaminophen, ascorbic acid, uric acid, lactate) at physiological maximums. | Sensor response for each interferent relative to the target analyte response. | Selectivity coefficient; demonstration of minimal cross-reactivity. |
| Anti-Biofouling Coating Efficacy | Incubating coated sensors in complex media (e.g., serum, plasma) and monitoring signal drift over time. | Signal stability, reduction in non-specific adsorption (measured via fluorescence/ELISA). | Comparison of performance between coated and uncoated sensors; microscopy for surface fouling [54]. |
| MIP-based Acetaminophen Detection | Electropolymerization of MIP on a nanomaterial-modified electrode, followed by template removal and electrochemical detection. | Limit of Detection (LOD), Linear Range, Selectivity against structural analogues. | Testing in real samples (serum, urine) with standard addition method; comparison to HPLC [55]. |
The Scientist's Toolkit: Essential Research Reagents & Materials
Table 2: Key Research Reagent Solutions for Sensor Development
| Reagent / Material | Function in Experimentation | Specific Example & Rationale |
|---|---|---|
| Molecularly Imprinted Polymer (MIP) | Provides high-selectivity recognition for a specific molecule (e.g., glucose, acetaminophen), filtering out interferents. | Polyresorcinol-based MIP for acetaminophen; offers a synthetic, stable, and selective alternative to biological receptors [55]. |
| MXene Nanosheets (e.g., Ti3C2Tx) | Enhances electrochemical signal transduction due to high conductivity and large surface area. | Ti3C2Tx nanosheets serve as an excellent platform for MIP attachment, improving sensor sensitivity and response time [55]. |
| Porous Carbon from ZIF-8 | Prevents the restacking of 2D materials (e.g., MXenes), maintaining high surface area and providing more active sites. | C-ZIF-8 crosslinks MXene nanosheets, forming a stable 3D network that enhances the electrochemical performance of the composite sensor [55]. |
| Cross-linked Protein Nanocomposite | Serves as a robust anti-biofouling coating to prevent non-specific protein and cell attachment on implantable sensors. | A coating of cross-linked BSA with pentaamine-functionalized reduced graphene oxide resists biofouling, extending functional sensor life in vivo [54]. |
| Permselective Membranes | Acts as a barrier to selectively control the diffusion of molecules to the sensor's transducer surface. | Membranes like Nafion or proprietary polymer "domains" can be tuned to block negatively charged or large molecules like common interferents [4]. |
Visualizing Workflows and Mechanisms
Sensor Interference Mechanism
MIP Sensor Development Workflow
Validation and Comparative Analysis of Commercial and Novel Biosensor Platforms
Comparative Profiling of Market-Leading CGM Systems and Their Labeled Interferents
Troubleshooting Guides
FAQ: What are the most common substances that interfere with CGM readings and why?
Answer: The most common interfering substances are acetaminophen, ascorbic acid (Vitamin C), and hydroxyurea. The interference occurs due to the core electrochemical sensing principle of most CGM systems. These devices use an enzyme, typically glucose oxidase, to catalyze a reaction with glucose in the interstitial fluid. This reaction generates a small electrical current that the sensor translates into a glucose reading. Chemically similar substances can also participate in or disrupt this electrochemical reaction, leading to a signal that does not accurately reflect the true glucose concentration [4] [56].
- Acetaminophen is electroactive and can be directly oxidized at the sensor's electrode, generating an additional current that is misinterpreted as coming from glucose [13].
- Ascorbic Acid (Vitamin C) is a strong reducing agent. It can donate electrons to the sensor's electrode, artificially increasing the measured current and leading to falsely elevated glucose readings [4] [56].
- Hydroxyurea was identified through post-market surveillance. Its specific mechanism is less published, but it is known to cause a positive bias, resulting in CGM readings that are higher than the actual blood glucose level [4] [56].
FAQ: How have manufacturers designed CGMs to mitigate these interferences?
Answer: Manufacturers have employed several design strategies, primarily through the use of advanced membrane technologies, to reduce the impact of interfering substances.
- Permselective Membranes: Dexcom incorporated a permselective membrane in its G6 and G7 models. This membrane is designed to selectively block the diffusion of specific molecules, like acetaminophen, based on their size and charge, preventing them from reaching the working electrode [4] [56].
- Bioprotective Membranes: These outer membranes serve a dual purpose: they promote biocompatibility to reduce the body's foreign body response, and they can also act as an initial barrier to limit the flux of interfering substances into the sensor's core [4].
- Biosensor Generation Selection: The choice of electrochemical biosensor design inherently changes the interference profile. First-generation biosensors (e.g., Dexcom, Medtronic) use oxygen as a natural electron acceptor and can be susceptible to electroactive interferents. Second-generation biosensors (e.g., Abbott FreeStyle Libre) use an artificial mediator, which allows for a lower operating voltage that minimizes the oxidation of common interferents like acetaminophen, though they may be susceptible to others like ascorbic acid [4].
Experimental Protocols
Detailed Methodology: Assessing Acetaminophen Interference in an Outpatient Setting
This protocol is based on a study designed to investigate the challenges to closed-loop systems [13].
1. Objective: To quantify the magnitude and duration of falsely elevated CGM glucose values following acetaminophen ingestion in an outpatient setting using contemporary sensor technology.
2. Materials and Reagents:
- CGM System: Dexcom G4 Platinum CGM System.
- Reference Glucose Meter: Bayer CONTOUR NEXT blood glucose meter.
- Interferent: 1000 mg acetaminophen tablets.
- Study Participants: 40 subjects with diabetes (28.5 ± 8.4 years, HbA1c 7.3 ± 0.8%).
3. Experimental Workflow:
4. Key Procedures:
- Baseline Measurement: Obtain simultaneous CGM and blood glucose (BG) meter readings immediately before acetaminophen ingestion (time 0).
- Dosing: Participants ingest a 1000 mg dose of acetaminophen at breakfast.
- Serial Blood Glucose Monitoring: Participants obtain BG meter readings at 0.5, 1, 2, 4, 6, and 8 hours post-ingestion. The CGM values are recorded at corresponding times.
- Data Adjustment: CGM glucose values are adjusted for baseline bias by correcting for the difference between the 0-minute CGM and BG meter values.
- Exclusion Criteria: Any CGM data following a sensor calibration during the 8-hour observation period are excluded to prevent confounding.
5. Outcome Measures:
- The primary outcome is the difference (least squares means and 95% CIs) between bias-adjusted CGM glucose values and BG meter values at each time point.
- The results demonstrated significant differences for all 8 hours after ingestion, with the greatest mean difference of 61 mg/dL (upper 95% CI of 77 mg/dL) occurring at 120 minutes [13].
Detailed Methodology: In-Vitro Investigation of Membrane Solutions for Acetaminophen Exclusion
This protocol is based on foundational research into eliminating acetaminophen interference [3].
1. Objective: To evaluate the efficacy of composite polymer membranes in eliminating acetaminophen interference for an implantable glucose sensor.
2. Materials and Reagents:
- Glucose Sensor: Implantable amperometric biosensor based on glucose oxidase.
- Polymer Materials: Cellulose acetate, Nafion, and a composite membrane of cellulose acetate and Nafion.
- Analytes: Glucose, acetaminophen, and other potential electrochemical interferents.
- Buffer Solution: Standard physiological buffer for in-vitro testing.
3. Experimental Workflow:
4. Key Procedures:
- Sensor Preparation: Fabricate sensors with different inner membrane configurations (e.g., cellulose acetate, Nafion, and a composite of both).
- Glucose Response Calibration: Immerse the sensor in a buffer solution and introduce known concentrations of glucose. Measure the steady-state current response (primarily from generated Hâ‚‚Oâ‚‚) to establish sensor sensitivity and linearity.
- Interference Challenge: In the same system, introduce physiologically relevant concentrations of acetaminophen and other interferents. Measure the sensor's current response.
- Performance Metrics: Evaluate membranes based on:
- Steady-state sensitivity to acetaminophen: The ideal membrane shows a significantly reduced or near-zero response.
- Response time to acetaminophen: A slow response, combined with the body's rapid clearance of acetaminophen, can make the interference clinically irrelevant.
- Preserved Hâ‚‚Oâ‚‚ diffusivity: The membrane must still allow the hydrogen peroxide signal from the glucose reaction to pass through efficiently [3].
5. Outcome Measures:
- The composite cellulose acetate/Nafion membrane was found to effectively eliminate acetaminophen interference while maintaining reasonable diffusivity for hydrogen peroxide, leading to excellent in vivo performance [3].
Data Presentation
Table 1: Labeled Interfering Substances for Market-Leading CGM Systems
Table summarizing manufacturer-labeled interfering substances and their effects as derived from user guides and regulatory documents [4].
| Manufacturer & CGM Model(s) | Biosensor Generation | Interfering Substance | Labeled Effect on Glucose Reading | Recommended Action / Threshold |
|---|---|---|---|---|
| Dexcom G6/G7/ONE | First | Acetaminophen | Falsely elevated | >1000 mg every 6 hours may increase readings |
| First | Hydroxyurea | Falsely elevated | Use blood glucose meter for treatment decisions | |
| Medtronic Guardian Connect / Simplera | First | Acetaminophen | Falsely elevated | May falsely raise readings; level of inaccuracy varies |
| First | Hydroxyurea | Falsely elevated | Do not use CGM if hydroxyurea is taken | |
| Abbott FreeStyle Libre 2/3 | Second | Ascorbic Acid (Vitamin C) | Falsely elevated | >500 mg per day may affect readings |
| Abbott FreeStyle Libre 14 day | Second | Ascorbic Acid (Vitamin C) | Falsely elevated | Taking vitamin C may falsely raise readings |
| Second | Salicylic Acid (Aspirin) | Falsely lowered | May slightly lower sensor glucose readings | |
| Senseonics Eversense | Optical | Tetracycline | Falsely lowered | Antibiotics of tetracycline class may lower readings |
| Optical | Mannitol/Sorbitol | Falsely elevated (IV) | May elevate readings when administered intravenously |
Table 2: The Scientist's Toolkit: Key Research Reagents & Materials
Essential materials for studying and mitigating interferent effects in glucose biosensors.
| Item | Function / Relevance in Interference Research |
|---|---|
| Acetaminophen | A primary electroactive interferent used to challenge first-generation biosensor designs and test the efficacy of blocking membranes [13] [3]. |
| Ascorbic Acid (Vitamin C) | A key reducing interferent used to evaluate the susceptibility of second-generation biosensors and assess membrane selectivity [4] [56]. |
| Hydroxyurea | An important pharmacologically relevant interferent, often identified via post-market surveillance; used for real-world performance testing [4] [56]. |
| Glucose Oxidase Enzyme | The core biological recognition element in most CGM systems. Understanding its kinetics is fundamental to modeling interference [4]. |
| Permselective Membranes (e.g., Nafion, Cellulose Acetate) | Polymer membranes engineered to selectively allow the passage of Hâ‚‚Oâ‚‚ while blocking larger or differently charged interfering molecules. Critical for sensor design improvements [4] [3]. |
| Bioprotective Membranes | Outer membrane domains designed to be biocompatible and to control the overall flux of substances from the interstitial fluid into the sensor, influencing interferent access [4]. |
Visualization of Core Concepts
CGM Biosensor Generations and Interference Mechanisms
FAQ: Core Technical Principles
Q1: What is the fundamental electrochemical difference between first and second-generation biosensor architectures?
A1: The core difference lies in the electron transfer mechanism from the enzyme to the electrode surface [4]:
- First-Generation: These biosensors rely on oxygen (Oâ‚‚) as a natural mediator. Oxygen shuttles electrons from the reduced glucose oxidase (GOx) enzyme to the electrode surface, producing hydrogen peroxide (Hâ‚‚Oâ‚‚) as a by-product. The subsequent oxidation of Hâ‚‚Oâ‚‚ is often measured to determine glucose concentration.
- Second-Generation: These systems replace oxygen with an artificial, synthetic redox mediator. This mediator facilitates electron transfer at a lower operating potential, which helps reduce the interference from other easily oxidizable substances, such as acetaminophen and ascorbic acid [4].
Q2: Why does acetaminophen interfere more significantly with first-generation CGM systems?
A2: Acetaminophen is an easily oxidizable substance. In first-generation biosensors, the high operating potential required to measure the hydrogen peroxide signal also readily oxidizes any acetaminophen that diffuses to the electrode. This concurrent oxidation generates an additional, non-glucose-related current, which the sensor misinterpretes as a falsely high glucose reading [8]. Second-generation biosensors, by operating at a lower potential optimized for the artificial mediator, are less likely to oxidize acetaminophen, thus minimizing this interference [4].
Q3: What design features do modern CGMs use to mitigate interferent effects?
A3: Manufacturers incorporate multiple design strategies to improve specificity [4]:
- Permselective Membranes: Specialized polymer membranes (e.g., Nafion) are coated onto the sensor to selectively block or reduce the flux of interfering substances like acetaminophen while allowing glucose to pass.
- Bioprotective Membranes: These outer membranes provide biocompatibility and offer an additional diffusion barrier to interferents.
- Interference Domain: A specific membrane layer designed to scavenge or block common interfering substances before they reach the sensing electrode.
Troubleshooting Guide: Acetaminophen Interference
Symptom
Sensor glucose readings are persistently and inexplicably elevated compared to fingerstick blood glucose measurements, following the administration of acetaminophen.
Investigation & Diagnosis
- Verify Suspected Interferent: Confirm the use of any medication containing acetaminophen. Note that acetaminophen is found in many over-the-counter cold, flu, and pain relief formulations.
- Check Manufacturer's Labeling: Consult the specific user guide for your biosensor model. The level of interference varies by manufacturer and sensor generation.
- Identify Sensor Architecture: Determine whether you are using a first or second-generation biosensor system to understand the inherent risk level. The table below provides a comparative overview.
Table 1: Head-to-Head Comparison of Biosensor Generations and Acetaminophen Interference
| Feature | First-Generation Biosensors | Second-Generation Biosensors |
|---|---|---|
| Electron Transfer Mechanism | Uses oxygen (Oâ‚‚) as the natural mediator [4] | Uses an artificial redox mediator [4] |
| Example CGM Systems | Dexcom G6/G7, Medtronic Guardian/Simplera [4] | Abbott FreeStyle Libre series [4] |
| Operating Potential | High (to oxidize Hâ‚‚Oâ‚‚) [8] | Low (optimized for the artificial mediator) [4] |
| Acetaminophen Interference | Significant, causes falsely elevated readings [4] [8] | Less susceptible; not a listed interferent for major models [4] |
| Primary Labeled Interferents | Acetaminophen, Hydroxyurea [4] [22] | Ascorbic Acid (Vitamin C) [4] [22] |
| Key Mitigation Strategy | Incorporation of permselective interference membranes (e.g., in Dexcom G6/G7) [4] | Use of a ferrocene-based mediator and membrane systems [4] |
Resolution & Best Practices
- For Research Settings: If your experiment involves acetaminophen, select a second-generation biosensor architecture or a system with validated interference-blocking membranes.
- Sensor Data Interpretation: Be aware of the pharmacodynamics of acetaminophen. Intravenous administration can cause a rapid spike in sensor discrepancy, peaking around 30 minutes post-administration, with a larger effect observed at lower glucose levels [8].
- Experimental Control: When designing protocols, include control measurements with a reference method (e.g., blood glucose meter) to quantify and correct for potential interference.
Experimental Protocols for Interference Quantification
Protocol 1: In-Vitro Characterization of Acetaminophen Interference
Objective: To quantitatively assess the cross-reactivity of acetaminophen on a biosensor platform.
Workflow: The following diagram illustrates the experimental setup and data analysis workflow.
Materials & Reagents:
- Biosensor System: Sensor array or commercial CGM unit.
- Analytes: D-Glucose, Acetaminophen (APAP).
- Buffer: Phosphate Buffered Saline (PBS), pH 7.4.
- Equipment: Potentiostat (for custom sensors), data logging software.
Procedure:
- Prepare a series of glucose solutions in PBS covering the physiological range (e.g., 2-20 mM).
- Spike each glucose solution with increasing concentrations of acetaminophen (e.g., 0, 50, 100, 200 µM).
- Expose the biosensor to each solution and record the steady-state output current.
- Calculate the percentage increase in signal attributed to acetaminophen at each glucose level.
- Validate the true glucose concentration in key samples using a reference method like HPLC.
Protocol 2: Electrode Area Ratio Optimization for Sensitivity
Objective: To optimize the counter-to-working electrode area ratio to improve sensor sensitivity and performance, a key step in developing robust biosensors [16].
Workflow: This experiment involves fabricating and testing sensors with different geometric configurations.
Materials & Reagents:
- Electrode Fabrication: SU-8 microneedle arrays, Ti/Pt and Ag for metalization [16].
- Functionalization: Glucose Oxidase (GOx), Bovine Serum Albumin (BSA), Glutaraldehyde, Nafion [16].
- Equipment: Potentiostat, microfabrication facilities (e.g., for DRIE).
Procedure:
- Fabricate sensor arrays with varying counter-to-working electrode area ratios (e.g., 1:1, 1:2, 1:3) [16].
- Functionalize the working electrode with GOx using BSA and glutaraldehyde as cross-linking agents [16].
- Apply a protective Nafion coating to the electrode surface.
- Characterize each sensor configuration in a glucose solution.
- Calculate the sensitivity (current density per mM glucose), Limit of Detection (LoD), and Limit of Quantification (LoQ) for each design. As demonstrated in one study, optimization from a 1:1 to a 1:3 ratio can improve sensitivity from 0.63 µA/mm²mM to 1.28 µA/mm²mM [16].
The Scientist's Toolkit: Research Reagent Solutions
Table 2: Essential Materials for Biosensor Development and Interference Testing
| Research Reagent / Material | Function in Experimentation |
|---|---|
| Glucose Oxidase (GOx) | The primary biological recognition element for glucose biosensing. It catalyzes the oxidation of glucose [16]. |
| Nafion | A perfluorinated polymer used as a permselective membrane coating. It reduces the flux of anionic interferents like ascorbic acid and uric acid to the electrode surface [16]. |
| Glutaraldehyde & BSA | Used as a cross-linking system to immobilize the GOx enzyme onto the electrode surface, ensuring stability and retention of enzymatic activity [16]. |
| Artificial Redox Mediators (e.g., Ferrocene derivatives) | Core component of second-generation biosensors. They shuttle electrons from GOx to the electrode at a lower applied potential, reducing interference [4]. |
| Acetaminophen (APAP) | A critical chemical interferent used for in-vitro validation of biosensor specificity and to test the efficacy of blocking membranes [16] [8]. |
Benchmarking Novel Sensor Platforms Against Reference Analytical Methods
A core challenge in the development of implantable biosensors, particularly for continuous health monitoring, is their susceptibility to interference from common substances. Acetaminophen, a widely used over-the-counter analgesic, is a classic and clinically significant interferent. It is known to cause falsely elevated glucose readings in many continuous glucose monitoring (CGM) systems, which could lead to dangerous clinical decisions [22] [4]. This technical support center is designed within the context of a broader thesis focused on mitigating this specific problem. The following guides and FAQs will provide researchers and scientists with a structured framework for benchmarking novel sensor platforms against established reference methods, with a specific emphasis on evaluating and overcoming acetaminophen interference.
Frequently Asked Questions (FAQs) for Researchers
1. Why is acetaminophen a particularly problematic interferent for electrochemical biosensors?
Acetaminophen is electroactive and can be oxidized at a similar potential to hydrogen peroxide (Hâ‚‚Oâ‚‚), which is a critical product of the glucose oxidase (GOx) enzyme reaction used in many first-generation biosensors. When acetaminophen reaches the working electrode, it contributes an additional, non-glucose-related current, leading to a falsely high glucose signal [4]. This is a fundamental design challenge for amperometric biosensors.
2. What are the key differences in how leading CGM designs manage interferent flux?
Manufacturers employ different membrane "domains" or layers to control the passage of substances from the interstitial fluid to the electrode surface. Key functional layers include:
- Interference Membrane: Designed to be selectively permeable, reducing the diffusion of common interferents like acetaminophen and ascorbic acid to the working electrode [4].
- Bioprotective Membrane: Serves as the primary contact with the biological environment, providing biocompatibility and acting as a physical barrier to biofouling and larger molecules, while also influencing the passage of interfering species [4].
3. How do "first-generation" and "second-generation" biosensor designs differ in their fundamental susceptibility to interferents?
The core distinction lies in their electron transfer mechanism, which dictates their operational voltage and thus the range of substances that might be oxidized and cause interference.
- First-Generation (e.g., Dexcom, Medtronic): Rely on oxygen as a natural electron acceptor. This design often requires a relatively high operating potential, making it susceptible to electroactive interferents like acetaminophen and hydroxyurea [4].
- Second-Generation (e.g., Abbott FreeStyle Libre): Use an artificial mediator to shuttle electrons. This allows for a significantly lower operating potential, thereby avoiding the oxidation of many common interferents. However, they can be susceptible to other substances, such as ascorbic acid (Vitamin C), which can reduce the mediator [4].
4. What are the critical parameters for benchmarking sensor performance against reference methods like HPLC?
When validating a novel sensor, especially for claims of reduced interference, its performance must be quantitatively compared to a reference standard. Key benchmarking parameters derived from validation experiments include [57] [58]:
- Allan Deviation: Measures bias stability and identifies the dominant noise types (e.g., velocity random walk, bias instability) over different averaging times.
- Hysteresis: Quantifies the maximum difference in sensor output when the target analyte concentration is approached from a lower vs. a higher value.
- Non-repeatability: Assesses the precision of the sensor by measuring the standard deviation of multiple measurements under the same conditions.
- Limit of Detection (LOD) & Limit of Quantification (LOQ): The lowest concentration of an analyte that can be reliably detected and quantified, respectively.
- Offset Temperature Stability: Evaluates how the sensor's baseline signal drifts with changes in ambient temperature.
Troubleshooting Guides
Guide 1: Designing an Experiment to Quantify Acetaminophen Interference
Objective: To systematically evaluate the effect of a specific interferent (e.g., acetaminophen) on the accuracy of a novel biosensor platform.
Summary of Workflow: The following diagram illustrates the core experimental workflow for interference testing.
Experimental Protocol:
- Sensor Preparation: Calibrate the novel sensor according to its standard protocol. Simultaneously, prepare the reference analytical system (e.g., HPLC, mass spectrometry) [59].
- Solution Preparation: Prepare a series of buffer solutions with a fixed, physiologically relevant concentration of glucose (e.g., 100 mg/dL).
- Baseline Measurement: Immerse the sensor in the glucose-only solution and record the stable output signal. Take a sample for analysis with the reference method to confirm the true glucose concentration.
- Introduction of Interferent: To the same solution, sequentially add known amounts of acetaminophen stock solution to achieve clinically relevant concentrations (e.g., from 5 mg/L to 50 mg/L, covering therapeutic and overdose levels) [4]. Allow the sensor signal to stabilize after each addition.
- Data Collection: Record the sensor's output signal at each acetaminophen concentration. Concurrently, take samples for immediate analysis with the reference method to establish the "true" glucose concentration.
- Data Analysis: Calculate the percentage error or absolute difference between the sensor reading and the reference method value at each interferent concentration. Perform statistical analysis (e.g., a t-test) to determine if the observed bias is significant.
Guide 2: Benchmarking Sensor Performance and Stability
Objective: To characterize the intrinsic noise, stability, and precision of a novel sensor platform, independent of specific interferents.
Experimental Protocol:
- Static Setup: Place the sensor in a controlled environment (e.g., a climatic chamber) with a constant analyte concentration and stable temperature [58].
- Long-Term Data Acquisition: Collect sensor output data at its maximum sampling rate for an extended period (typically 4-8 hours) without any changes to the environment [58].
- Allan Deviation Analysis: Process the collected data by calculating the Allan deviation. This analysis helps identify the optimal averaging time for the sensor and quantifies its fundamental noise components, such as bias instability and random walk [58].
- Hysteresis Testing: Using a precision test bench, expose the sensor to a full-cycle of analyte concentrations (e.g., low -> high -> low). Record the output at each step. The hysteresis error is calculated as the maximum deviation between the up-cycle and down-cycle measurements at the same concentration point [58].
- Repeatability Testing: Under identical conditions, repeatedly measure the sensor's response to the same analyte concentration. The non-repeatability is typically reported as the standard deviation of this series of measurements [58].
Table 1: Documented Interference Profiles of Marketed CGM Systems
This table summarizes labeled interfering substances for commercially available sensors, providing a benchmark for research targets [22] [20] [4].
| Manufacturer & Model | Biosensor Generation | Key Labeled Interfering Substances | Effect on Sensor Reading |
|---|---|---|---|
| Dexcom (G6, G7, ONE+) | First | Acetaminophen (>1g/6hr in adults), Hydroxyurea | Falsely elevates readings |
| Medtronic (Guardian 4, Simplera) | First | Acetaminophen (any dose), Hydroxyurea | Falsely elevates readings |
| Abbott (FreeStyle Libre 2/3) | Second | Ascorbic Acid/Vitamin C (>500 mg/day) | Falsely elevates readings |
| Abbott (FreeStyle Libre 14 day) | Second | Ascorbic Acid, Salicylic Acid (Aspirin) | Elevates readings, Slightly lowers readings |
| Senseonics Eversense | Optical | Tetracycline, IV Mannitol/Sorbitol | Falsely lowers, Falsely elevates readings |
Table 2: Key Parameters for Benchmarking Sensor Performance
This table outlines critical metrics and methodologies for a comprehensive sensor performance evaluation, based on standardized benchmarking practices [57] [58].
| Performance Parameter | Description | Experimental Method | Ideal Outcome |
|---|---|---|---|
| Allan Deviation | Characterizes bias stability and noise over time. | Long-term data collection in a static, controlled setting. | A clear peak identifying the bias instability and a low noise floor. |
| Hysteresis | Maximum output difference when approaching a concentration from opposite directions. | Full-cycle concentration testing (low-high-low). | < 1% of full-scale output. |
| Non-Repeatability | Standard deviation of multiple measurements under identical conditions. | Repeated measurement of a fixed analyte concentration. | A low standard deviation, indicating high precision. |
| Limit of Detection (LOD) | Lowest analyte concentration that can be reliably detected. | Signal-to-noise ratio (S/N=3) analysis in dose-response experiments. | A value significantly lower than the intended clinical measurement range. |
| Offset Temperature Stability | Drift of the baseline signal with temperature changes. | Measurement in a climatic chamber across a relevant temperature range (e.g., 20°C to 40°C). | Minimal drift per degree Celsius of temperature change. |
The Scientist's Toolkit: Research Reagent Solutions
This table lists essential materials and their functions for conducting the experiments described in the troubleshooting guides.
| Item | Function in Research | Example / Note |
|---|---|---|
| Glucose Oxidase (GOx) | Biological recognition element; catalyzes the oxidation of glucose, producing Hâ‚‚Oâ‚‚. | The most common enzyme in first-generation electrochemical glucose biosensors [4]. |
| Permselective Membranes | Coating on the electrode surface designed to be selectively permeable; blocks interferents while allowing glucose passage. | A key design feature in Dexcom G6/G7 to reduce acetaminophen interference [4]. |
| Artificial Mediators (e.g., Ferrocene derivatives) | Shuttles electrons from the enzyme's reaction center to the electrode in second-generation biosensors. | Allows for a lower operating potential, reducing susceptibility to acetaminophen [4]. |
| Bioprotective Membranes | Outer membrane that provides biocompatibility, prevents biofouling, and modulates mass transport. | Critical for the longevity and stability of implantable sensors in vivo [6]. |
| Hydrogel Matrix | A water-swollen polymer network that can host enzymes and other sensing components; regulates diffusion. | Often used to encapsulate the sensing chemistry, improving stability and biocompatibility [60]. |
| Acetaminophen Analytical Standard | A high-purity compound used to prepare precise stock solutions for interference testing. | Essential for creating dose-response curves in interference experiments. |
| Phosphate Buffered Saline (PBS) | A stable, isotonic buffer solution to maintain a constant pH and ionic strength during in-vitro testing. | Mimics the salt composition of interstitial fluid. |
Frequently Asked Questions (FAQs)
Q1: What fundamentally differentiates the Sinocare iCan i3's third-generation biosensor design from earlier CGM models in terms of interference?
The Sinocare iCan i3 employs a third-generation electrochemical biosensor design, which represents a significant architectural shift from dominant first and second-generation continuous glucose monitoring (CGM) systems. [4]
The table below summarizes the core differentiating characteristics:
| Feature | First-Generation CGM (e.g., Dexcom, Medtronic) | Second-Generation CGM (e.g., FreeStyle Libre) | Third-Generation CGM (Sinocare iCan i3) |
|---|---|---|---|
| Electron Transfer Mechanism | Relies on oxygen as a natural mediator. [4] | Uses an artificial mediator species. [4] | Direct electron transfer from the enzyme cofactor to the electrode surface. [4] |
| Primary Interference Risk | Electroactive substances (e.g., acetaminophen, hydroxyurea) that oxidize at the working electrode. [4] [8] | Substances that interact with the artificial mediator. [4] | Engineered to minimize mediator-related interference; manufacturer claims no acetaminophen or vitamin C interference. [4] |
| Operating Potential | Higher operating potentials, increasing susceptibility. [8] | Reduced operating potentials. [4] | Not specified, but designed for targeted electron transfer. |
This direct electron transfer mechanism is the foundational reason for the manufacturer's claims of reduced susceptibility to common electroactive interfering substances like acetaminophen and vitamin C. [4]
Q2: What is the manufacturer's stated position on substance interference for the iCan i3 sensor?
According to the manufacturer's claims, identified from owner's booklets and marketing materials, the Sinocare iCan i3 system has a specific interference profile. [4]
The key stated positions are summarized in the table below:
| Claim Category | Manufacturer's Stated Position |
|---|---|
| Acetaminophen & Vitamin C | No acetaminophen or vitamin C interference. [4] |
| External Impurities & Oxygen | Not susceptible to interference from external impurities. No oxygen interference. [4] |
| Other Conditions/Medications | It is not known how different conditions or medications may affect system performance. The device should not be used if the patient is pregnant, on dialysis, implanted with a pacemaker, or is critically ill. [4] |
Q3: What are the critical considerations for designing an experiment to independently verify the iCan i3's interference claims?
Independent verification of interference claims requires a robust experimental design that accounts for the complexities of in vivo measurement. The following protocol outlines a methodological approach.
Experimental Workflow for Interference Verification
Phase 1: In Vitro Testing in Surrogate Interstitial Fluid (ISF)
- Objective: To establish a baseline interference effect in a controlled matrix.
- Methodology: Spike a surrogate ISF solution with physiological concentrations of glucose and the potential interferent (e.g., acetaminophen at high therapeutic levels of 1000 mg/L). The CGM sensor's response is compared against a reference method (e.g., YSI glucose analyzer). The test should follow guidelines from CLSI EP07 for interference testing. [61]
- Data Analysis: Calculate the bias against a control sample without the interferent. Per ISO 15197, a bias exceeding ±0.55 mmol/L (±10 mg/dL) at glucose levels <5.55 mmol/L (<100 mg/dL) or ±10% at higher levels indicates significant interference. [61]
Phase 2: In Vivo Clinical Study
- Objective: To assess interference in the intended use environment.
- Methodology: Administer the potential interferent (e.g., 1000 mg acetaminophen orally or intravenously) to study participants wearing the iCan i3 sensor. [8] Measure capillary or venous blood glucose frequently as a reference.
- Data Analysis: Use statistical metrics like Mean Absolute Relative Difference (MARD) to compare CGM readings from ISF against blood glucose reference values. A significant increase in MARD or a consistent directional bias (e.g., falsely elevated readings) after interferent administration confirms an interference effect. [8]
Q4: Why is the concentration of an interferent in interstitial fluid (ISF) a major challenge in CGM research?
The primary challenge is the difficulty in directly measuring and quantifying analyte concentrations in ISF. [61]
While it is reasonable to assume that substances present in blood will also be found in ISF, their absolute concentrations may differ significantly due to physiological processes like paracellular diffusion and transcellular transport. [61] However, ISF is challenging to extract in volumes sufficient for analysis with standard reference instruments. [61] Consequently, researchers and CGM developers often report interference effects based on substance levels in blood plasma or spiked into surrogate ISF, which may not accurately reflect the true clinical environment the sensor operates in. [61] This knowledge gap underscores the need for advanced techniques, such as microdialysis, to better characterize the pharmacokinetics of interferents in ISF. [61]
The Scientist's Toolkit: Key Reagents & Materials
The table below lists essential materials and their functions for conducting interference experiments on implantable glucose sensors.
| Item | Function & Application in Research |
|---|---|
| Surrogate Interstitial Fluid (ISF) | A chemically defined solution mimicking the ionic and protein composition of ISF. Used for controlled in vitro calibration and interference screening. [61] |
| Potential Interferents | Pharmaceutical-grade substances (e.g., Acetaminophen, Ascorbic Acid, Hydroxyurea) for spiking into surrogate ISF or administering in clinical studies to test sensor specificity. [4] [8] |
| Reference Glucose Analyzer | A high-precision instrument (e.g., YSI Life Sciences analyzer) considered a "gold standard" for providing true glucose values against which CGM accuracy is compared. [61] |
| Microdialysis System | A catheter-based system for direct in vivo sampling of ISF in volumes sufficient for independent analysis. Helps bridge the gap between blood and ISF interferent concentrations. [61] |
| Data Analysis Software | Statistical software (e.g., R, Python) for calculating key performance metrics like MARD, bias, and conducting regression analyses to quantify interference effects. [8] |
Interference Mechanism Comparison: Sensor Generations
Analyzing the Unique Interference Profile of the Eversense Optical Sensing System
Frequently Asked Questions (FAQs)
Q1: What is the core technological difference between the Eversense E3 CGM system and most other continuous glucose monitors?
A1: The Eversense E3 CGM system uses a unique fluorescent technology instead of the electrochemical-enzymatic sensing principle used by most other transcutaneous CGM systems [62] [63]. Its fully implantable sensor contains an abiotic (non-enzyme based), fluorescent glucose-indicating polymer that reacts to glucose levels in the interstitial fluid [62]. The fundamental recognition reaction is a reversible condensation of the cis-diol groups of glucose with the bis-boronate moieties of the indicator polymer. Glucose binding disrupts intramolecular fluorescence quenching, resulting in an increase in fluorescence intensity proportional to glucose concentration [62]. This optical mechanism is fundamentally different from enzymatic systems that measure an electrical current from a glucose oxidation reaction.
Q2: Why is the Eversense system unaffected by common interferents like acetaminophen and ascorbic acid (Vitamin C)?
A2: The Eversense system is unaffected because its sensing mechanism does not rely on electrochemical reactions that can be disrupted by easily oxidized substances [62] [4]. Electrochemical CGM sensors, which use glucose oxidase and measure a resulting electrical current, can be interfered with by substances like acetaminophen because these substances are also oxidized at the sensor's electrode, producing a false additional signal [8]. Since the Eversense sensor uses optical fluorescence and contains no electrode or enzyme, it is not subject to these specific interference mechanisms [62]. Potential interferents for Eversense would be substances that bind to its polymer or fluoresce light in its specific operational spectrum.
Q3: Are there any substances known to interfere with the Eversense E3 CGM System?
A3: Yes, in-vitro testing has identified two primary classes of substances that can interfere with the Eversense sensor [62] [4]:
- Tetracycline: Antibiotics of the tetracycline class may falsely lower sensor glucose readings [4].
- Mannitol/Sorbitol: May falsely elevate sensor readings when administered intravenously or as a component of an irrigation solution. It is important to note that typical dietary intake of sorbitol from artificial sweeteners does not impact sensor accuracy [4].
Troubleshooting Guide: Investigating Unexplained Sensor Readings
Scenario: A research subject wearing an Eversense E3 sensor presents with a sensor glucose reading that does not match their symptomatic experience or a fingerstick blood glucose measurement.
| Step | Action | Rationale & Technical Notes |
|---|---|---|
| 1 | Confirm Calibration | Ensure the sensor was calibrated with a highly accurate blood glucose meter (BGM). The performance of the Eversense E3 is dependent on the quality of the BGM used for its required calibrations [63]. |
| 2 | Review Medication Log | Systematically review the subject's recent medication and supplement administration. Focus on identifying the use of tetracycline antibiotics or the intravenous administration of mannitol or sorbitol [4]. |
| 3 | Verify Sensor Location & Site Health | Assess the sensor insertion site for signs of infection, inflammation, or scar tissue. While the sensor is implanted, local tissue reactions can theoretically affect performance. |
| 4 | Check for External Physical Pressure | Inquire about any prolonged physical pressure on the transmitter area (e.g., during sleep). Compression can sometimes cause transiently inaccurate readings. |
| 5 | Contact Manufacturer Support | If no cause is identified, contact Senseonics technical support for further diagnostics and to report a potential device performance issue. They may request data logs for analysis. |
Experimental Protocols for Assessing Sensor Interference
For researchers aiming to validate or explore the interference profile of optical glucose sensors, the following in-vitro methodology, adapted from published literature on the Eversense system, provides a robust framework [62].
Protocol: In-Vitro Interference Screening for Fluorescent Glucose Sensors
Objective: To characterize the interference profile of a fluorescent glucose sensor against a panel of endogenous and exogenous substances.
Methodology Summary: A paired-sample method adapted from the Clinical and Laboratory Standards Institute (CLSI) guidance document EP7-A2 [62].
Key Reagents and Materials:
- Test Sensor: Eversense (or other fluorescent glucose-sensing polymer).
- Reference Analyzer: A clinical-grade plasma glucose analyzer (e.g., YSI).
- Interference Substances: A panel of 40+ substances, including vicinal diols (monosaccharides, disaccharides, catechols, alpha-hydroxy carboxylic acids, aminosugars) and common drugs like acetaminophen and ascorbic acid [62].
- Glucose Solutions: Prepared at least two different glucose concentrations (e.g., ~75 mg/dL and ~320 mg/dL) [62].
- Test Medium: A suitable buffer or matrix that mimics interstitial fluid.
Procedure:
- Baseline Measurement: Immerse the sensor in the test medium with a known glucose concentration and record the stable fluorescent signal. Simultaneously, measure the glucose concentration with the reference analyzer.
- Introduction of Interferent: Add the test substance to the medium at a supratherapeutic/supraphysiologic plasma concentration.
- Post-Interference Measurement: Record the sensor's glucose reading after the introduction of the interferent and measure the actual glucose concentration with the reference analyzer.
- Bias Calculation: Calculate the sensor bias using the formula:
Sensor Bias = (Sensor Glucose Reading) - (Reference Plasma Glucose Concentration). - Significance Threshold: A substance is considered an interferent if the calculated sensor bias exceeds the limits set by the International Organization for Standardization (ISO) 15197:2013 [62].
- Dose-Response Follow-up: For any substance producing a significant bias, perform a dose-response test to determine the concentration at which the bias becomes significant relative to therapeutic ranges.
The workflow for this experimental protocol is outlined below.
Comparative Interference Data
The table below summarizes quantitative data from in-vitro interference testing of the Eversense system, clearly demonstrating its resilience to common interferents like acetaminophen and ascorbic acid at physiologic concentrations [62].
Table 1: In-Vitro Interference Screening Results for Eversense CGM System [62]
| Substance | Maximum Therapeutic Plasma Concentration | Tested Concentration | Sensor Bias at ~75 mg/dL Glucose | Sensor Bias at ~320 mg/dL Glucose | Clinically Significant? |
|---|---|---|---|---|---|
| Acetaminophen | 3.0 mg/dL | 20 mg/dL | -8.7 mg/dL | -8.3% | No |
| Ascorbic Acid (Vitamin C) | 2.0 mg/dL | 6.0 mg/dL | +7.7 mg/dL | +0.1% | No |
| Tetracycline | Data in source | Data in source | Exceeded ISO limits | Exceeded ISO limits | Yes |
| Mannitol | Data in source | Data in source | Exceeded ISO limits | Exceeded ISO limits | Yes |
| L-DOPA | 0.4 mg/dL | 1.2 mg/dL | -20.0 mg/dL | -11.0% | No |
For context, the following table compares the labeled interfering substances for various CGM systems based on first-generation (electrochemical) and the unique Eversense (optical) sensing principles [22] [4].
Table 2: Comparison of Manufacturer-Labeled Interfering Substances Across CGM Platforms
| CGM Model | Biosensor Generation | Key Interfering Substances & Labeling Notes |
|---|---|---|
| Dexcom G6/G7 | First (Electrochemical) | Acetaminophen (>1g/6hrs may raise readings); Hydroxyurea (raises readings) [4]. |
| Medtronic Guardian 4/Simplera | First (Electrochemical) | Acetaminophen (any dose may falsely raise readings); Hydroxyurea (do not use) [4]. |
| FreeStyle Libre 2/3 | Second (Electrochemical) | Ascorbic Acid (>500 mg/day may raise readings) [22] [4]. |
| Senseonics Eversense | Optical (Non-electrochemical) | Tetracycline (may lower readings); Mannitol/Sorbitol (IV administration may raise readings) [4]. |
The Scientist's Toolkit: Key Research Reagents
Table 3: Essential Materials for Investigating CGM Interference Profiles
| Reagent / Material | Function in Experimental Context |
|---|---|
| Fluorescent Glucose Sensor (Eversense) | The device under test (DUT); the implantable component containing the glucose-indicating polymer [62] [63]. |
| Reference Plasma Glucose Analyzer (e.g., YSI) | Provides the "ground truth" glucose measurement against which sensor accuracy and bias are calculated [62]. |
| Acetaminophen & Ascorbic Acid Solutions | Common positive controls for electrochemical sensor interference; used as negative controls to demonstrate the robustness of optical sensing [62]. |
| Tetracycline & Mannitol Solutions | Known positive control interferents for the Eversense optical sensor system [62] [4]. |
| Vicinal Diol Compound Library | A panel of substances (e.g., monosaccharides, catechols, sugar alcohols) structurally related to glucose, which have a higher potential for cross-reactivity with the boronic acid moieties in the Eversense polymer [62]. |
| Simulated Interstitial Fluid (Buffer) | Provides a consistent and physiologically relevant in-vitro medium for testing sensor performance and interference [62]. |
Signaling Pathway and Mechanism Visualization
The core signaling mechanism of the Eversense E3 CGM system, which underlies its unique interference profile, is illustrated in the diagram below.
Conclusion
The challenge of acetaminophen interference is a multi-faceted problem demanding an integrated approach spanning fundamental electrochemistry, advanced materials science, and clinical awareness. Key takeaways include the proven clinical relevance of the interference, the effectiveness of engineered membrane systems and electrochemical methods for its mitigation, and the critical need for standardized, transparent interference testing for all new sensor platforms. Future directions must focus on the development of next-generation biosensing elements less prone to common interferents, the creation of universal testing protocols, and the implementation of real-time correction algorithms. For researchers and drug developers, success hinges on a proactive design philosophy that prioritizes sensor specificity and safety in the context of polypharmacy, thereby unlocking the full potential of implantable biosensors for personalized medicine.
References
- 1. Elimination of electrochemical interferences in glucose ... [https://www.sciencedirect.com/science/article/abs/pii/S0165993610000269]
- 2. Evaluating in vitro and in vivo the interference of ascorbate ... [https://www.sciencedirect.com/science/article/abs/pii/095656639285030E]
- 3. Elimination of the acetaminophen interference in an ... - PubMed [https://pubmed.ncbi.nlm.nih.gov/8160962/]
- 4. Part 2: Marketed CGM Designs, Labeled Interfering ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12531190/]
- 5. Amperometric Enzyme-based Biosensors for Lowering the ... [https://www.intechopen.com/chapters/6809]
- 6. Recent Progress and Challenges of Implantable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11051912/]
- 7. Material Design in Implantable Biosensors toward Future ... [https://www.mdpi.com/2076-3417/13/7/4630]
- 8. Interference of Intravenous Acetaminophen with ... [https://www.jmaj.jp/detail.php?id=10.31662%2Fjmaj.2025-0186]
- 9. Interference of Intravenous Acetaminophen with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12598134/]
- 10. Biocompatibility of common implantable sensor materials ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6767110/]
- 11. Medications That Interfere With Continuous Glucose ... [https://www.meded101.com/medications-that-interfere-with-continuous-glucose-monitoring/]
- 12. Frontiers | Recent advances in glucose monitoring utilizing oxidase electrochemical biosensors integrating carbon-based nanomaterials and smart enzyme design [https://www.frontiersin.org/journals/chemistry/articles/10.3389/fchem.2025.1591302/full]
- 13. Effect of Acetaminophen on CGM Glucose in an Outpatient ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4876736/]
- 14. Direct Evidence of Acetaminophen Interference with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4717519/]
- 15. A novel conductive membrane sensor protection technique ... [https://www.sciencedirect.com/science/article/pii/S2590137023000638]
- 16. Electrode area ratio optimization and interferometric ... [https://www.sciencedirect.com/science/article/abs/pii/S0026265X25009129]
- 17. In Vitro, In Vivo and Post Explantation Testing of Glucose- ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC1987311/]
- 18. Implantable biosensor and methods of use thereof [https://patents.google.com/patent/US8914090B2/en]
- 19. Dynamic Interference Testing—Unexpected Results ... [https://www.mdpi.com/1424-8220/25/7/1985]
- 20. 7 Factors That Affect Your CGM Accuracy [https://www.snaq.ai/blog/7-factors-that-affect-your-cgm-accuracy-sensor-placement-medications-and-more]
- 21. Two chromatographic methods for analyzing paracetamol ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11791058/]
- 22. CGM Interfering Substances Cheat Sheet [https://diabetesed.net/cgm-interfering-substances-cheat-sheet/]
- 23. Electrochemical Impedance Spectroscopy-Based ... [https://www.mdpi.com/2079-6374/15/7/443]
- 24. Starch-Based Electrochemical Sensors and Biosensors [https://link.springer.com/article/10.1007/s44174-022-00012-5]
- 25. a state-of-the-art review and future perspectives [https://enveurope.springeropen.com/articles/10.1186/s12302-025-01138-1]
- 26. Addressing the Selectivity of Enzyme Biosensors: Solutions ... [https://www.mdpi.com/1424-8220/21/9/3038]
- 27. A critical review in the clinical field [https://www.sciencedirect.com/science/article/abs/pii/S0003267024010444]
- 28. Smartphone Biosensors for Non-Invasive Drug Monitoring ... [https://www.mdpi.com/2079-6374/15/3/163]
- 29. Advances of Nanozyme-Driven Multimodal Sensing ... [https://www.mdpi.com/2079-6374/15/6/375]
- 30. Intelligent nanozymes: Biomimetic design, mechanisms ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12327865/]
- 31. Versatile biomimetic catalyst functionalized nanozymes for ... [https://www.sciencedirect.com/science/article/abs/pii/S1385894723052221]
- 32. Weaving an “electronic spider†web [https://www.sciencedirect.com/science/article/abs/pii/S0026265X2503293X]
- 33. Improving the performance of biosensors: Developing new ... [https://www.sciencedaily.com/releases/2025/01/250130140825.htm]
- 34. Biosensors, Artificial Intelligence Biosensors, False Results ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12025796/]
- 35. Biocompatible Coatings - Applications in Biomedical ... [https://hydromer.com/biocompatible-coatings/]
- 36. Pressure Sensor Drift: Causes, Consequences, and Prevention [https://www.measurex.com.au/blog/pressure-sensor-drift-causes-consequences-and-prevention/]
- 37. Drift in Analog Sensors: Why Signal Accuracy Declines ... [https://gesrepair.com/drift-in-analog-sensors-why-signal-accuracy-declines-over-time]
- 38. Analog Sensor Woes: Dealing with Drift, Noise, and Non- ... [https://runtimerec.com/analog-sensor-woes-dealing-with-drift-noise-and-non-linearity/]
- 39. Skin-Integrated Wearable Systems and Implantable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7400150/]
- 40. Long-term drift behavior in metal oxide gas sensor arrays [https://www.nature.com/articles/s41597-025-05993-8]
- 41. Reduction of acetaminophen interference in glucose ... [https://link.springer.com/article/10.1007/BF00403381]
- 42. Three-dimensional highway-like graphite flakes/carbon ... [https://www.sciencedirect.com/science/article/pii/S2590049822000340]
- 43. Low-cost fiber-based electrochemical sensor for ... [https://www.nature.com/articles/s44294-025-00088-6]
- 44. Optimization and characterization of a lactate-oxidase electrode [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra07173a]
- 45. Does acetaminophen alter the glucose readings of ... [https://www.freestyle.abbott/eg-en/support/faq/question-answer.html?q=the-freestyle-libre-system-tab-question-17]
- 46. Laboratory Protocol and Pilot Results for Dynamic ... [https://pubmed.ncbi.nlm.nih.gov/35549522/]
- 47. Troubleshooting and Optimization Tips for SPR Experiments [https://www.creative-proteomics.com/resource/troubleshooting-optimization-tips-for-spr-experiments.htm]
- 48. In Vitro Diagnostic Regulation (IVDR)—Frequently Asked ... [https://www.thermofisher.com/us/en/home/clinical/ivdr/faqs.html]
- 49. Current state of the art and future directions for implantable ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC10539032/]
- 50. Reduction of acetaminophen interference in glucose sensors ... [https://pubmed.ncbi.nlm.nih.gov/7926347/]
- 51. Analytical techniques for the determination of acetaminophen [https://www.sciencedirect.com/science/article/abs/pii/S016599361830267X]
- 52. Electrochemical Detection of Acetaminophen in ... [https://www.mdpi.com/2227-9040/12/7/122]
- 53. Voltammetric determination of acetaminophen in ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9420686/]
- 54. Biosensors, Volume 15, Issue 3 (March 2025) – 72 articles [https://www.mdpi.com/2079-6374/15/3]
- 55. Electrochemical Detecting of Acetaminophen Based on the ... [https://www.sciencedirect.com/science/article/abs/pii/S235249282501894X]
- 56. Interferences With CGM Systems: Practical Relevance? [https://pmc.ncbi.nlm.nih.gov/articles/PMC8861798/]
- 57. Implementation and benchmarking of a novel analytical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC6143516/]
- 58. Characterization and Benchmark of a Novel Capacitive ... [https://www.mdpi.com/1424-8220/21/23/8030]
- 59. Prospective analytical role of sensors for environmental ... [https://www.sciencedirect.com/science/article/abs/pii/S0165993622002345]
- 60. Implantable electrochemical biosensors: Challenges, ... [https://www.sciencedirect.com/science/article/pii/S2451910325001048]
- 61. Classification of Continuous Glucose Monitoring Devices ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12614895/]
- 62. Interference Assessment of Various Endogenous and ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC5963543/]
- 63. Learn the Science Behind the CGM Accuracy & Data ... [https://www.eversensecgm.com/resources-e3/long-term-exceptional-accuracy/]