Advanced Acetylcholinesterase Immobilization Techniques for Next-Generation Electrochemical Biosensors
This article provides a comprehensive analysis of contemporary strategies for immobilizing acetylcholinesterase (AChE) onto electrode surfaces, a critical technology for developing sensitive and stable biosensors.
Advanced Acetylcholinesterase Immobilization Techniques for Next-Generation Electrochemical Biosensors
Abstract
This article provides a comprehensive analysis of contemporary strategies for immobilizing acetylcholinesterase (AChE) onto electrode surfaces, a critical technology for developing sensitive and stable biosensors. Tailored for researchers, scientists, and drug development professionals, the content explores foundational principles, diverse methodological approaches from physical adsorption to covalent bonding on nanostructured platforms, and essential optimization techniques to enhance biosensor performance. It further delivers a critical evaluation of validation protocols and comparative assessments of different immobilization techniques, highlighting their applications in pesticide detection, clinical diagnostics, and therapeutic agent screening. The synthesis of this information aims to serve as a definitive guide for the rational design and implementation of AChE-based biosensing platforms in both research and industrial settings.
Acetylcholinesterase Fundamentals and Immobilization Rationale for Biosensor Design
The Critical Role of AChE in Neurotransmission and as a Biorecognition Element
Biological Function of Acetylcholinesterase in Neurotransmission
Acetylcholinesterase (AChE; EC 3.1.1.7) is a crucial serine hydrolase enzyme concentrated at neuromuscular junctions and cholinergic brain synapses, where it serves a vital function in terminating nerve impulses by catalyzing the hydrolysis of the neurotransmitter acetylcholine (ACh) [1] [2]. This rapid hydrolysis, which occurs in microseconds, ensures the precise control of synaptic transmission necessary for proper muscle function and cognitive processes [1].
The enzyme's catalytic mechanism involves an active site containing a catalytic triad of serine, histidine, and glutamate residues [2]. The hydrolysis reaction proceeds through a two-step process: First, ACh binds to the enzyme's active site, where the serine residue undergoes nucleophilic attack on the carbonyl carbon of acetylcholine. This results in the cleavage of the ester bond, releasing choline and forming an acetylated enzyme intermediate. Second, the acetyl group is rapidly hydrolyzed, releasing acetic acid and regenerating the free enzyme [1]. This process maintains the delicate balance of neurotransmitter levels essential for normal nervous system function.
Figure 1: AChE in Neurotransmission. AChE terminates neuronal signaling by hydrolyzing acetylcholine (ACh) in the synaptic cleft, allowing for signal precision and choline recycling.
When AChE activity is inhibited by toxic compounds such as organophosphorus pesticides or nerve agents, acetylcholine accumulates in the synaptic cleft, leading to continuous stimulation of muscles and glands [1] [3]. This cholinergic hyperexcitability manifests as a range of symptoms from headache and confusion to respiratory failure and death, underscoring the critical importance of AChE in maintaining neurological homeostasis [1].
AChE as a Biorecognition Element in Analytical Applications
The exceptional specificity of AChE for its substrate, coupled with its sensitivity to inhibition by various toxic compounds, makes it an excellent biorecognition element in biosensor technology [4] [5]. AChE-based biosensors have emerged as ultra-sensitive and rapid analytical tools for toxicity monitoring in environmental, food, and clinical applications [5].
The fundamental principle underlying these biosensors involves measuring the decrease in AChE activity upon exposure to inhibitors, which is directly proportional to the concentration of the toxicant present [1]. In a typical configuration, the biosensor consists of AChE immobilized on a transducer surface. When the substrate acetylthiocholine is introduced, the enzymatic reaction produces electroactive thiocholine, which generates a measurable signal. In the presence of inhibitors, this signal decreases due to the reduced enzymatic activity [1] [6].
Detection capabilities of AChE-based biosensors span multiple classes of toxic compounds:
Table 1: Analytical Performance of AChE-Based Biosensors for Various Analytes
| Analyte Class | Specific Examples | Detection Limit | Linear Range | Application Context |
|---|---|---|---|---|
| Organophosphorus Insecticides | Paraoxon, Dichlorvos, Chlorpyrifos ethyl oxon | 10⁻⁸ to 10⁻⁹ M [7] | Not specified | Environmental monitoring, food safety [7] |
| Carbamate Pesticides | Carbofuran, Aldicarb | Not specified | Not specified | Agricultural monitoring [5] |
| Chemical Warfare Agents | Sarin, Soman, Tabun, VX | 7.41×10⁻¹² M (Sarin) to 6.17×10⁻¹¹ M (Tabun) [3] | 10⁻¹¹ to 10⁻⁴ M [3] | Defense, security, emergency response [3] |
| Heavy Metals | Arsenic(III), Mercury(II) | 1.1×10⁻⁸ M (As³⁺) [6] | 10⁻⁸ to 10⁻⁷ M [6] | Environmental water monitoring [6] |
| Natural Toxins | Aflatoxins | Not specified | Not specified | Food quality control [4] |
The versatility of AChE biosensors extends to multiple transduction methods, including amperometric, potentiometric, colorimetric, and fluorometric systems, each offering distinct advantages for specific applications [2] [5]. Recent advances have incorporated nanomaterials and recombinant enzyme engineering to enhance sensitivity, selectivity, and stability [1] [4].
Experimental Protocols for AChE Immobilization and Biosensor Application
AChE Immobilization on Screen-Printed Electrodes: Comparative Methods
The immobilization of AChE onto transducer surfaces represents a critical step in biosensor fabrication, significantly influencing performance parameters including sensitivity, stability, response time, and reproducibility [1] [7]. Below are three validated protocols for AChE immobilization on screen-printed electrodes (SPEs):
Protocol 1: Entrapment in a Photopolymerisable Polymer (PVA-SbQ)
- Material Preparation: Prepare a solution containing AChE (0.8-1.2 mIU) in 0.1 M phosphate buffer (pH 7.4). Mix thoroughly with poly(vinyl alcohol) bearing styrylpyridinium groups (PVA-SbQ) polymer precursor.
- Electrode Modification: Deposit 2-5 μL of the enzyme-polymer mixture onto the working electrode surface of SPEs.
- Photopolymerization: Expose the modified electrode to UV light (λ = 360 nm) for 5 minutes to achieve cross-linking and polymer formation.
- Rinsing and Storage: Rinse the biosensor with phosphate buffer to remove unentrapped enzyme. Store at 4°C in dry conditions when not in use [7].
Protocol 2: Bioencapsulation in Sol-Gel Composite
- Sol-Gel Preparation: Mix 200 μL of methyltrimethoxysilane (MTMOS) with 60 μL of HCl (1 mM) and sonicate for 30 seconds until a clear solution forms.
- Enzyme-Ink Formulation: Combine the sol-gel solution with AChE (0.8-1.2 mIU), graphite powder, hydroxyethyl-cellulose (1% w/v), and polyethylene glycol 600 (1% w/v) to form a homogeneous ink.
- Electrode Printing: Apply the enzyme-containing ink to the working electrode area of SPEs using screen-printing technology.
- Curing: Allow the biosensor to cure at room temperature for 24 hours to complete the gelation process [7].
Protocol 3: Covalent Immobilization via Metal-Chelate Affinity
- Surface Functionalization: Modify the electrode surface with a chelating agent (e.g., iminodiacetic acid) and charge with Ni²⁺ ions.
- Enzyme Engineering: Utilize recombinant AChE-(His)₆ from Drosophila melanogaster containing a hexahistidine tag.
- Enzyme Attachment: Incubate the functionalized electrode with the modified AChE solution for 60 minutes at 25°C to allow specific metal-chelate coordination.
- Washing: Rinse thoroughly with phosphate buffer to remove non-specifically bound enzyme [7].
Table 2: Performance Comparison of AChE Immobilization Methods
| Immobilization Method | Apparent Km (mM) | Storage Stability | Relative Activity Retention | Advantages | Limitations |
|---|---|---|---|---|---|
| Photopolymerisable Polymer (PVA-SbQ) | 0.67 [7] | >6 months [7] | High (>80%) [7] | Rapid process, good enzyme activity retention | Potential enzyme leakage, diffusion barriers |
| Sol-Gel Encapsulation | 0.32 [7] | >6 months [7] | Moderate to high [7] | Mild preparation conditions, tunable porosity | Brittleness, possible mass transfer limitations |
| Metal-Chelate Affinity | 0.45 [7] | Not specified | High [7] | Oriented immobilization, minimized enzyme denaturation | Requires engineered enzyme, complex preparation |
| Gelatin-Glutaraldehyde Cross-linking | Not specified | >1 month at 4°C [3] | High [3] | Simple protocol, low cost | Potential over-crosslinking reducing activity |
Protocol for Detection of Organophosphorus Compounds Using AChE Biosensors
Principle: Organophosphorus (OP) compounds irreversibly inhibit AChE, reducing the enzymatic conversion of acetylthiocholine to thiocholine, which is electrochemically detectable [1] [7].
Materials Required:
- AChE biosensor (prepared according to above protocols)
- Phosphate buffered saline (PBS, 0.1 M, pH 7.4)
- Acetylthiocholine chloride substrate solution (10 mM in PBS)
- Standard solutions of OP compounds (paraoxon, dichlorvos, etc.)
- Electrochemical workstation with chronoamperometric capability
Procedure:
- Biosensor Conditioning: Hydrate the AChE biosensor in PBS for 5 minutes before use.
- Inhibition Phase: Incubate the biosensor in sample solution (or standard OP solutions) for 20 minutes at 25°C.
- Measurement Phase: Transfer the biosensor to an electrochemical cell containing 800 μL PBS. Add 100 μL of acetylthiocholine solution (final concentration 1 mM).
- Signal Detection: Apply a potential of +640 mV (vs. Ag/AgCl) and measure the oxidation current of thiocholine for 60 seconds [7] [3].
- Data Analysis: Calculate percentage inhibition using the formula: % Inhibition = [(I₀ - I)/I₀] × 100, where I₀ is the current from uninhibited biosensor and I is the current from sample-exposed biosensor.
Validation Parameters:
- Limit of detection: 10⁻⁸ to 10⁻⁹ M for paraoxon [7]
- Linear range: 10⁻¹⁰ to 10⁻⁶ M [7]
- Reproducibility: RSD <4% [6]
- Total analysis time: <25 minutes
Figure 2: AChE Inhibition Assay Workflow. The protocol for detecting cholinesterase inhibitors based on enzyme activity measurement following exposure to sample.
The Researcher's Toolkit: Essential Reagents and Materials
Table 3: Key Research Reagent Solutions for AChE Biosensor Development
| Reagent/Material | Function/Application | Example Specifications | Technical Notes |
|---|---|---|---|
| Acetylcholinesterase (AChE) | Biorecognition element | Electric eel AChE, 16.7 μkat/mg [3]; Recombinant AChE from D. melanogaster [7] | Source affects sensitivity to inhibitors [5] |
| Acetylthiocholine chloride | Enzyme substrate | 10 mM in buffer [3] | Electroactive product (thiocholine) enables amperometric detection |
| Screen-printed electrodes (SPEs) | Transducer platform | Pt working electrode (diameter 1 mm), Pt auxiliary, Ag/AgCl reference [3] | Enable mass production, disposable use [7] [6] |
| Glutaraldehyde | Cross-linking agent | 1% (w/v) in immobilization matrix [3] | Stabilizes immobilized enzyme; concentration critical to prevent over-crosslinking |
| Methyltrimethoxysilane (MTMOS) | Sol-gel precursor | Mixed with HCl (1 mM) for hydrolysis [7] | Forms porous matrix for enzyme encapsulation |
| Poly(vinyl alcohol) bearing styrylpyridinium groups (PVA-SbQ) | Photopolymerizable matrix | UV light (λ = 360 nm) for 5 min cross-linking [7] | Enables rapid enzyme entrapment under mild conditions |
| Phosphate buffered saline (PBS) | Measurement buffer | 0.1 M, pH 7.4 [3] | Optimal for AChE activity and stability |
| 5,5'-dithiobis(2-nitrobenzoic acid) (DTNB) | Colorimetric reagent | Ellman's method for activity assessment [7] | Produces yellow anion for spectrophotometric detection |
Core Principles of Electrochemical AChE Biosensors and Inhibition-Based Detection
Electrochemical biosensors based on acetylcholinesterase (AChE) inhibition represent a powerful analytical platform for detecting various toxic compounds, including organophosphorus pesticides and chemical warfare agents. These biosensors function on the principle that specific inhibitors permanently block AChE's catalytic activity, enabling highly sensitive quantification of these analytes. The core mechanism involves immobilizing AChE on an electrode surface, where it hydrolyzes the substrate acetylthiocholine (ATCh) to produce thiocholine, an electroactive product that generates a measurable current. When AChE is inhibited, the production of thiocholine decreases, resulting in a diminished electrochemical signal proportional to the inhibitor concentration [8] [6].
The performance of these biosensors critically depends on two fundamental aspects: the method employed to stably attach AChE to the electrode surface while preserving its biological activity, and the choice of electrode materials that enhance electron transfer and signal amplification. Recent advancements have incorporated innovative nanomaterials and immobilization strategies to achieve unprecedented sensitivity and stability, pushing detection limits to sub-parts-per-billion levels for certain analytes [8] [9] [10]. This document provides a comprehensive technical resource for researchers developing these biosensing platforms, featuring detailed protocols, performance comparisons, and essential methodological considerations framed within contemporary research on AChE immobilization techniques.
Core Principles and Signaling Mechanisms
The operational principle of AChE biosensors relies on the enzyme-catalyzed hydrolysis of acetylthiocholine chloride (ATCl) and the subsequent electrochemical detection of the reaction product. In the uninhibited state, AChE catalyzes the hydrolysis of ATCl to produce thiocholine (TCh) and acetic acid. The generated TCh is then electrochemically oxidized at the electrode surface, typically at a defined applied potential. This oxidation reaction produces a measurable amperometric current that serves as the baseline signal [8] [6].
When organophosphorus compounds (OPs) or other AChE inhibitors are present, they phosphorylate or carbamylate the serine residue within the enzyme's active site, leading to irreversible inhibition. This modification drastically reduces the enzyme's catalytic activity, resulting in decreased TCh production and a consequent reduction in the oxidation current. The percentage of enzyme inhibition is quantitatively related to the inhibitor concentration and can be calculated using the formula: % Inhibition = [(I₀ - I)/I₀] × 100, where I₀ is the steady-state current before inhibition and I is the current after inhibition and incubation [6]. This relationship forms the quantitative foundation for analyte detection, with the incubation time being a critical parameter influencing sensitivity.
Table 1: Key Reaction Steps in AChE Inhibition Biosensing
| Step | Process | Description | Output |
|---|---|---|---|
| 1 | Enzyme Reaction | AChE hydrolyzes acetylthiocholine chloride | Thiocholine + Acetic Acid |
| 2 | Electrochemical Detection | Thiocholine oxidation at electrode surface | Measurable current signal |
| 3 | Inhibition Mechanism | Organophosphorus compounds phosphorylate AChE | Irreversible enzyme inactivation |
| 4 | Signal Measurement | Reduced thiocholine production decreases current | Quantitative inhibition reading |
Diagram 1: AChE Biosensor Inhibition Mechanism
Performance Comparison of AChE Biosensor Platforms
Recent research has demonstrated remarkable advancements in AChE biosensor technology through the implementation of novel nanomaterials and immobilization strategies. The integration of graphene oxide (GO), polyaniline (PANI), silver nanoparticles (AgNPs), and MXene quantum dots has yielded systems with exceptional sensitivity and wide linear dynamic ranges. These nanomaterials serve dual functions: providing high-surface-area scaffolds for efficient enzyme immobilization and enhancing electron transfer kinetics for signal amplification [8] [10].
The detection limits achieved by contemporary biosensor configurations surpass those of conventional analytical methods for certain applications, with some platforms capable of detecting organophosphorus compounds at concentrations as low as 1.07 × 10⁻⁶ ppb for omethoate and 1 × 10⁻¹⁷ M for chlorpyrifos [8] [10]. The following table provides a comprehensive comparison of recently reported AChE biosensor platforms, highlighting their analytical performance characteristics for various target analytes.
Table 2: Performance Comparison of Advanced AChE Biosensor Platforms
| Sensor Architecture | Target Analyte | Linear Range | Detection Limit | Optimal Conditions | Reference |
|---|---|---|---|---|---|
| AChE/AgNPs/GO/PANI/SPCE | Dimethyl methylphosphonate (DMMP) | Not specified | 6.43 × 10⁻⁵ ppb | 0.1 M PBS, pH 7.5, 12-min incubation | [8] |
| AChE/AgNPs/GO/PANI/SPCE | Omethoate | Not specified | 1.07 × 10⁻⁶ ppb | 0.1 M PBS, pH 7.5, 12-min incubation | [8] |
| AChE/Ti₃C₂Tₓ MQDs/SPCE | Chlorpyrifos | 10⁻¹⁴ – 10⁻⁸ M | 1 × 10⁻¹⁷ M | Differential Pulse Voltammetry | [10] |
| AChE/SPCE (covalent) | Arsenic(III) | 1 × 10⁻⁸ to 1 × 10⁻⁷ M | 1.1 × 10⁻⁸ M | Britton-Robinson buffer pH 7, +0.6 V applied potential | [6] |
| Right-side-out RBCM-coated biosensor | Huperzine A | Not specified | 0.41 pmol/L | Immunoaffinity immobilization | [9] |
Beyond the analytical performance metrics, the storage stability and reproducibility of these biosensors represent critical parameters for practical implementation. Systems employing enzyme encapsulation in polymer films or sol-gel composites have demonstrated remarkable stability, maintaining functionality for over six months under proper storage conditions [7]. The reproducibility of fabrication processes is equally important, with reported relative standard deviation (RSD) values as low as 2.54% for multiple sensor batches, ensuring consistent performance across different production runs [8].
Detailed Experimental Protocols
Protocol 1: Detection of Organophosphorus Compounds Using Nanomaterial-Modified SPCE
Principle: This protocol describes the fabrication of a high-sensitivity AChE biosensor using graphene oxide (GO), polyaniline (PANI), and silver nanoparticles (AgNPs) co-modified screen-printed carbon electrodes (SPCE) for detecting organophosphorus compounds (OPs) based on enzyme inhibition [8].
Materials Required:
- Screen-printed carbon electrodes (SPCEs)
- Acetylcholinesterase (AChE) from Electrophorus electricus
- Graphene oxide (GO) suspension
- Aniline monomer for PANI synthesis
- Silver nitrate (AgNO₃) for AgNPs synthesis
- Dimethyl methylphosphonate (DMMP) and omethoate as standard OPs
- Acetylthiocholine chloride (ATCl) as enzyme substrate
- Phosphate buffer saline (PBS, 0.1 M, pH 7.5)
- Glutaraldehyde (25%) for cross-linking
Procedure:
- Electrode Modification:
- Synthesize PANI via chemical oxidation of aniline in acidic medium
- Prepare GO suspension through modified Hummers' method
- Electrodeposit AgNPs on SPCE at constant potential
- Apply GO/PANI composite onto AgNPs/SPCE using freeze-drying technique
- Characterize modified electrode using cyclic voltammetry (CV) in potassium ferrocyanide solution
Enzyme Immobilization:
- Prepare AChE solution (concentration optimized based on activity)
- Apply 8 μL AChE solution onto GO/PANI/AgNPs/SPCE surface
- Cross-link with 0.25% glutaraldehyde vapor for 15 minutes
- Rinse with PBS to remove unimmobilized enzyme
- Store at 4°C when not in use
Inhibition and Detection:
- Record baseline current in PBS containing ATCl (0.1-10 mM) using CV
- Incubate inhibited biosensor with OP sample for 12 minutes in PBS (pH 7.5)
- Measure current response after incubation
- Calculate inhibition percentage: % Inhibition = [(I₀ - I)/I₀] × 100
- Generate calibration curve by plotting % inhibition vs. OP concentration
Validation Parameters:
- Linear range: Verify across 3-5 orders of magnitude
- Detection limit: Calculate based on 3×standard deviation of blank/slope
- Reproducibility: Determine using RSD of 5 different sensors
- Selectivity: Test against potential interferents (heavy metals, other pesticides)
Protocol 2: MXene Quantum Dot-Based Ultrasensitive Biosensor for Pesticide Detection
Principle: This protocol utilizes Ti₃C₂Tₓ MXene quantum dots (MQDs) to create an ultrasensitive biosensing platform for detecting organophosphorus pesticides through cholinesterase inhibition, achieving exceptional detection limits via enhanced electron transfer and enzyme stabilization [10].
Materials Required:
- Ti₃AlC₂ MAX phase for MQD synthesis
- Hydrofluoric acid (HF) or lithium fluoride (LiF)/HCl etching solution
- Chitosan (CS) solution (0.5-1% in acetic acid)
- Acetylcholinesterase (500 U/mg)
- Glutaraldehyde (GA, 2.5% in PBS)
- Chlorpyrifos, acephate, glyphosate standards
- Phosphate buffer (0.1 M, pH 7.4)
Procedure:
- MQD Synthesis:
- Etch Ti₃AlC₂ MAX phase in HF or LiF/HCl at 35-45°C for 24-48 hours
- Wash repeatedly with deionized water until supernatant reaches pH ~6
- Perform hydrothermal treatment at 150-200°C for 10-24 hours to form MQDs
- Characterize MQDs using AFM, SEM, and XRD to confirm size (2-5 nm) and structure
Biosensor Fabrication:
- Prepare MQD-chitosan composite (1:3 ratio)
- Deposit 6-8 μL composite solution on clean GCE surface, dry at room temperature
- Apply AChE solution (0.5-2 U) onto MQD-CS/GCE
- Cross-link with 2.5% glutaraldehyde vapor for 30 minutes
- Rinse thoroughly with PBS to remove excess cross-linker
Electrochemical Measurement:
- Employ differential pulse voltammetry (DPV) from 0 to 0.8 V
- Use acetylthiocholine iodide (ACTI) as substrate at optimal concentration (2-5 mM)
- Record DPV response in blank solution (I₀) and after incubation with pesticide (I)
- Utilize standard addition method for real sample analysis
- Validate with in vitro cholinergic activity assays in biological samples
Critical Notes:
- MQD concentration significantly impacts sensitivity—optimize between 1-5 mg/mL
- Cross-linking time affects enzyme activity—test between 15-60 minutes
- Inhibition time directly correlates with sensitivity—standardize at 10-15 minutes
Diagram 2: SPCE Biosensor Fabrication Workflow
The Scientist's Toolkit: Essential Research Reagents and Materials
The development of high-performance AChE biosensors requires careful selection of materials and reagents that collectively determine the analytical characteristics of the final biosensing platform. The table below comprehensively lists essential research reagents, their specific functions, and considerations for selection and optimization.
Table 3: Essential Research Reagents for AChE Biosensor Development
| Reagent/Material | Function/Purpose | Key Considerations |
|---|---|---|
| Acetylcholinesterase | Biological recognition element | Source (electric eel, human recombinant), specific activity (>500 U/mg), stability |
| Screen-printed Carbon Electrodes | Disposable transducer platform | Low cost, mass production capability, customizable design |
| Graphene Oxide (GO) | Nanomaterial for signal amplification | High conductivity, large surface area, enhances electron transfer |
| Polyaniline (PANI) | Conducting polymer matrix | Improves electron transfer rate, stabilizes enzyme immobilization |
| Silver Nanoparticles | Signal enhancement | Biocompatibility, high conductivity, increases coupling probability with AChE |
| MXene Quantum Dots | Novel nanomaterial for ultrasensitive detection | Quantum confinement effects, superior conductivity, high surface-to-volume ratio |
| Acetylthiocholine chloride | Enzyme substrate | Electrochemical conversion to thiocholine, generates measurable signal |
| Glutaraldehyde | Cross-linking agent | Forms stable covalent bonds between enzyme and matrix, concentration critical |
| Chitosan | Biopolymer matrix | Excellent film-forming ability, biocompatibility, non-toxic |
| Phosphate Buffer Saline | Electrochemical measurement medium | Optimal pH (7.0-7.5) for AChE activity, ionic strength affects signal |
Advanced Immobilization Techniques and Methodological Considerations
Comparative Analysis of Immobilization Methods
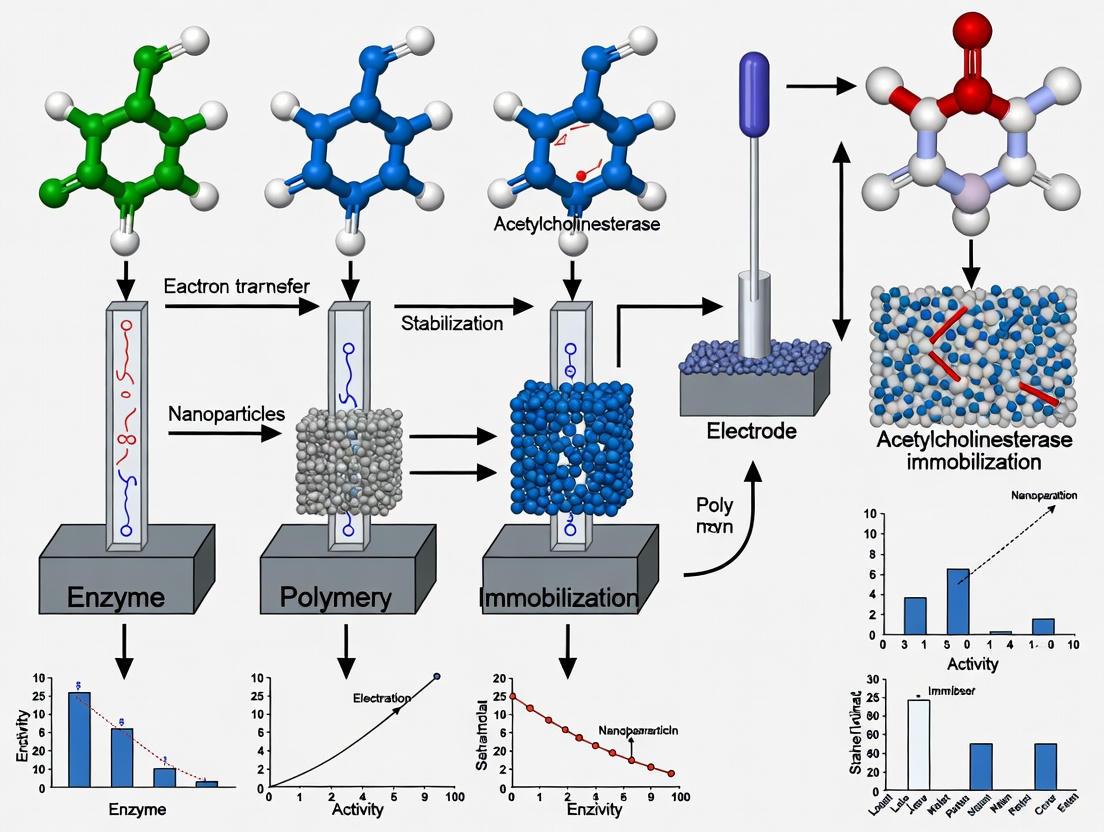
The strategic immobilization of AChE onto electrode surfaces represents a critical determinant of biosensor performance, influencing enzyme stability, activity retention, and overall sensor longevity. Research has systematically compared multiple immobilization approaches, revealing distinct advantages and limitations for each methodology [7].
Sol-Gel Encapsulation: This technique involves entrapping AChE within a porous silica matrix formed through the hydrolysis and condensation of alkoxide precursors. The sol-gel method provides exceptional storage stability exceeding six months, with demonstrated detection capabilities for paraoxon, dichlorvos, and chlorpyrifos ethyl oxon in the 10⁻⁸ to 10⁻⁹ M range following a 20-minute incubation period. The encapsulation process protects the enzyme from denaturation while allowing substrate and product diffusion, though potential limitations include reduced enzyme activity due to conformational constraints and slower response times resulting from additional diffusion barriers [7].
Metal-Chelate Affinity Immobilization: This approach utilizes engineered AChE containing polyhistidine tags (AChE-(His)₆) that specifically interact with metal ions (typically Ni²⁺ or Cu²⁺) coordinated on the electrode surface. The method enables oriented immobilization, potentially preserving more active sites accessible to substrate. Comparative studies have shown slightly improved Michaelis-Menten constants (Kₘᵃᵖᵖ = 0.45 mM) compared to other methods, indicating potentially better substrate accessibility. However, the requirement for enzyme engineering and possible metal ion leakage represent practical limitations for some applications [7].
Photopolymerizable Entrapment: This technique involves incorporating AChE within a poly(vinyl alcohol) bearing styrylpyridinium groups (PVA-SbQ) matrix that polymerizes upon UV light exposure. This method offers rapid and simple enzyme immobilization with minimal chemical modification, helping to preserve enzymatic activity. The approach yields favorable Michaelis-Menten constants (Kₘᵃᵖᵖ = 0.67 mM) and enables pesticide detection at nanomolar concentrations. The main advantages include simplicity and the ability to precisely pattern the enzyme layer, though potential photodamage to the enzyme during polymerization requires careful optimization of UV exposure conditions [7].
Emerging Orientation-Preserving Immobilization Strategies
Recent innovative approaches have focused on preserving the native orientation and membrane association of AChE to maintain its maximal biological activity. The development of right-side-out-oriented red blood cell membrane-coated electrochemical biosensors (ROCMCBs) represents a significant advancement in this direction. This methodology employs immunoaffinity interactions to orient red blood cell membranes on electrode surfaces, specifically exposing the extracellular domain of membrane-anchored AChE [9].
The ROCMCB platform demonstrates several advantages over conventional methods: it fully exposes AChE binding sites by controlling membrane orientation, maintains the enzyme's natural conformation as a peripheral membrane-anchoring protein, and preserves optimal interaction capabilities with inhibitors and substrates. This approach has enabled exceptional sensitivity for detecting AChE inhibitors like huperzine A, with limits of detection reaching 0.41 pmol/L. Furthermore, this bio-mimetic strategy has facilitated the rapid identification and evaluation of six potentially active anti-Alzheimer's compounds (baicalin, geniposide, gastrodin, berberine, rhynchophylline, and senkyunolide A) from traditional Chinese medicines, highlighting its utility in drug discovery applications [9].
The implementation of this orientation-controlled immobilization requires specific technical steps: (1) isolation of red blood cell membranes through sequential centrifugation and osmotic lysis; (2) antibody-mediated surface functionalization using CD47 intracellular primary antibody; (3) oriented assembly through immunoaffinity between gold-conjugated secondary antibodies and membrane-bound primary antibodies; and (4) comprehensive validation using immunofluorescence techniques with confocal laser scanning microscopy to confirm proper orientation [9].
This application note delineates the principal advantages of immobilized enzyme systems over their free enzyme counterparts, with a specific focus on stability, reusability, and operational practicality. Framed within the context of advanced acetylcholinesterase (AChE) biosensor research for drug development and diagnostic applications, the document provides a comparative quantitative analysis, detailed experimental protocols for common immobilization techniques on electrode surfaces, and essential resource guidance to facilitate robust biocatalytic system development. The integration of immobilized AChE is highlighted as a critical enabling technology for enhancing the performance and reliability of electrochemical biosensors in pharmaceutical and clinical settings.
Enzyme immobilization, defined as the confinement or localization of enzymes to a distinct region of space with retention of their catalytic activities, has evolved into a powerful tool for biocatalyst engineering [11]. This technology addresses inherent limitations of free enzymes in solution, including limited operational stability, short shelf-life, challenges in recovery and reuse, and poor compatibility with continuous industrial processes [12]. Within the specific research domain of acetylcholinesterase (AChE)-based biosensors, immobilization is not merely a convenience but a fundamental requirement for creating durable, sensitive, and reusable analytical devices for drug discovery, environmental monitoring, and clinical diagnostics [13] [14]. This note systematically outlines the key advantages, provides actionable protocols, and curates essential tools for researchers developing next-generation immobilized enzyme systems.
Comparative Advantages of Immobilized Enzymes
The transition from free to immobilized enzymes confers significant benefits that enhance both the enzyme's intrinsic properties and its process integration capabilities. The table below provides a structured comparison of these advantages.
Table 1: Key Advantages of Immobilized Enzymes over Free Enzymes
| Advantage Category | Key Metrics & Performance Outcomes | Relevance to AChE Biosensors |
|---|---|---|
| Enhanced Stability | Increased resistance to temperature, pH, and organic solvents [12] [15]. Retained activity over extended storage periods [13]. | AChE@MnMOF platform showed superior stability and resistance to harsh environments compared to free AChE [13]. |
| Reusability & Recovery | Capability for multiple use cycles (often 10+ cycles). Easy separation from reaction mixture, preventing product contamination [12] [16]. | Enables continuous or repeated batch operations; simplifies enzyme separation, reducing process costs [12]. |
| Process Control & Practicality | Suitable for continuous flow reactors. Improved control over reaction parameters. Reduced labor input and minimized reaction time [16] [17]. | Fundamental for constructing electrochemical sensors for repeated or continuous monitoring of anticholinesterase drugs or pesticides [14]. |
| Functional Efficiency | Potential for increased enzyme-substrate ratio. Improved specificity and product purity in some cases [17]. | Confinement on an electrode surface can enhance local substrate concentration and signal transduction efficiency [18]. |
| Economic Viability | Reduced enzyme consumption over time. Lower costs associated with production, labor, and downstream processing [16]. | Reuse of the biosensing interface drastically lowers the cost-per-analysis for high-throughput drug screening [19]. |
Experimental Protocols for Acetylcholinesterase Immobilization
The selection of an immobilization strategy is application-specific. Below are detailed protocols for two highly relevant techniques used in AChE biosensor fabrication: Covalent Binding on Metal-Organic Frameworks (MOFs) and Physical Adsorption on Prussian Blue-Modified Electrodes.
Protocol: Covalent Immobilization of AChE on a Squaric Acid-Based MnMOF Platform
This protocol describes a gentle, one-pot method for constructing an AChE-immobilized platform (AChE@MnMOF) that demonstrates superior stability for electrochemical sensing [13].
1. Research Reagent Solutions
- Squaric Acid Sodium Solution (0.1 M): Dissolve 0.1 M of squaric acid in 0.2 M NaOH solution using ultrasonication.
- Manganese Chloride Solution (0.1 M): Prepare in a 15% (v/v) ethanol solution.
- Acetylcholinesterase (AChE) Stock: Dissolve 1 mg of AChE in 0.5 mL of the prepared squaric acid sodium solution.
- Buffer: 0.1 M Phosphate Buffered Saline (PBS), pH 7.4.
2. Step-by-Step Workflow
- Enzyme-Carrier Mixing: Combine the 0.5 mL AChE-squaric acid solution with 0.5 mL of the 0.1 M manganese chloride tetrahydrate solution.
- Synthesis Incubation: Allow the mixture to stand undisturbed at room temperature for 60 minutes to facilitate the formation of the AChE@MnMOF composite.
- Washing and Harvesting: Centrifuge the suspension (e.g., 5000 rpm for 5 min) and carefully discard the supernatant. Wash the resulting AChE@MnMOF pellet three times with 0.1 M PBS (pH 7.4) to remove any unbound enzyme.
- Storage: Re-suspend the final AChE@MnMOF product in PBS (pH 7.4) and store at 4°C when not in use.
3. Critical Experimental Parameters
- pH: The reaction must be conducted under mild pH conditions to preserve enzyme activity.
- Temperature: Room temperature (approx. 25°C) synthesis is crucial to prevent enzyme denaturation.
- Control: Synthesize the MnMOF carrier without AChE in parallel to serve as a control for subsequent experiments.
The following diagram illustrates the experimental workflow and the charge repulsion sensing mechanism of the resulting AChE@MnMOF platform.
Protocol: Co-Immobilization of AChE and BChE via Adsorption for Broad-Spectrum Insecticide Screening
This protocol details the co-immobilization of AChE and Butyrylcholinesterase (BChE) on a modified electrode surface via adsorption and diazonium chemistry, enabling the broad-spectrum detection of insecticides [20].
1. Research Reagent Solutions
- Electrode Modifier: 4-aminothiophenol monolayer for diazonium chemistry.
- Prussian Blue Stabilizer: Copper-containing Prussian blue, electrodeposited on the modified electrode.
- Enzyme Solution: A mixture of AChE and BChE in a suitable buffer (e.g., Tris-HCl, pH 8.0).
- Buffer: 0.1 M Tris-HCl buffer, pH 8.0.
2. Step-by-Step Workflow
- Electrode Modification: Electrodeposit a stabilized, copper-containing Prussian blue layer onto an electrode pre-coated with a 4-aminothiophenol monolayer.
- Enzyme Immobilization: Drop-cast the solution containing both AChE and BChE onto the modified electrode surface.
- Adsorption Incubation: Incubate the electrode at 4°C for 12-24 hours to allow for physical adsorption and stable layer formation.
- Rinsing: Gently rinse the electrode with cold Tris-HCl buffer (pH 8.0) to remove any loosely bound enzyme molecules.
3. Critical Experimental Parameters
- Incubation Time: A prolonged incubation period (12-24 hours) is often necessary to achieve strong adsorption and minimize enzyme leaching.
- Temperature: Incubation at 4°C helps maintain enzyme stability during the process.
- Enzyme Ratio: The optimal ratio of AChE to BChE should be empirically determined for the target analytes.
The Scientist's Toolkit: Essential Research Reagent Solutions
Successful development of immobilized enzyme systems requires careful selection of materials. The following table catalogs key reagents and their functions in the context of AChE immobilization for biosensing.
Table 2: Essential Research Reagents for AChE Immobilization Studies
| Reagent / Material | Function & Rationale | Example from Literature |
|---|---|---|
| Metal-Organic Frameworks (MOFs) | Nano-porous carriers with high surface area for high enzyme loading; tailorable pore channels [13] [18]. | MnMOF with squaric acid ligand used for stable AChE immobilization under mild conditions [13]. |
| Prussian Blue & Derivatives | Inorganic matrices for electrode modification; excellent electrocatalytic properties and stability [20]. | Copper-containing Prussian blue used for co-immobilization of AChE and BChE [20]. |
| Chitosan & Other Natural Polymers | Biocompatible, biodegradable, and cost-effective supports with functional groups for covalent/ionic attachment [15]. | Chitosan is a popular choice due to its cationic nature and ease of functionalization. |
| Glutaraldehyde | A common homobifunctional crosslinker; forms Schiff bases with amino groups (e.g., lysine) on enzymes for covalent binding [15]. | Used as a linker molecule to activate carrier surfaces for stable covalent enzyme attachment [15]. |
| Acetylthiocholine (ATCh) | Synthetic substrate for AChE; enzymatic hydrolysis produces electroactive thiocholine (TCh+), enabling amperometric detection [13] [14]. | Used as the substrate in the AChE@MnMOF sensor; TCh+ production leads to a measurable charge repulsion effect [13]. |
| Ru(NH₃)₆³⁺ (Rubhex) | Positively charged electrochemical probe; used in "turn-on" sensing mechanisms based on charge repulsion with TCh+ [13]. | Served as the core signaling probe in the charge-repulsion-based AChE@MnMOF sensor [13]. |
The strategic relationships between these reagents and the core components of a biosensor are visualized below.
The immobilization of enzymes, particularly acetylcholinesterase, presents a transformative approach for advancing biosensor technology in drug development. The documented advantages in stability, reusability, and overall practicality underscore its critical role in transitioning from laboratory curiosities to robust, commercially viable analytical tools. The protocols and reagent solutions provided herein offer a foundational framework for researchers to design and implement high-performance immobilized enzyme systems, thereby accelerating innovation in pharmaceutical analytics and diagnostic applications.
The performance of an electrochemical biosensor is fundamentally dictated by the properties of the supporting material used to immobilize the biological recognition element. For acetylcholinesterase (AChE)-based biosensors, the support matrix must not only secure the enzyme but also actively preserve its activity and facilitate efficient electron transfer. Among the critical material properties, biocompatibility, specific surface area, and ease of functionalization emerge as three pillars essential for developing reliable and sensitive interfaces. This document details the core principles and experimental protocols for characterizing these properties, providing a framework for advancing AChE immobilization techniques in electrochemical biosensing.
Core Material Properties and Characterization
The interplay between a material's intrinsic properties dictates its suitability as a platform for AChE immobilization. The table below summarizes the key properties, their impact on biosensor function, and standard characterization techniques.
Table 1: Core Support Material Properties for AChE Immobilization
| Property | Impact on Biosensor Performance | Common Characterization Methods |
|---|---|---|
| Biocompatibility | Preserves enzymatic structure and activity; minimizes denaturation; ensures operational stability. [21] [13] | Enzyme activity assays; viability/staining assays for living cells; monitoring electrochemical signal retention over time. |
| High Surface Area | Increases enzyme loading capacity; enhances signal-to-noise ratio; improves catalytic efficiency. [21] [22] | Brunauer-Emmett-Teller (BET) analysis; Scanning Electron Microscopy (SEM). |
| Functionalization | Enables strong, stable enzyme attachment (e.g., covalent binding); introduces desired chemical groups for specific interactions. [23] [22] | Fourier Transform Infrared Spectroscopy (FTIR); X-ray Photoelectron Spectroscopy (XPS). |
Experimental Protocols for Material Evaluation
Protocol: Functionalization of Renewable Carbon via Acid Treatment
This protocol outlines the functionalization of renewable carbon (RC) to create a highly functionalized platform (RCF) ideal for AChE binding, based on a validated biosensor design. [23]
- Principle: Acid treatment with a sulfonitric mixture introduces oxygen-containing functional groups (e.g., carboxyl, hydroxyl) onto the carbon surface, enhancing its hydrophilicity and providing anchoring sites for enzyme immobilization.
Materials:
- Renewable Carbon (RC) powder, derived from biomass pyrolysis. [23]
- Nitric Acid (HNO₃) and Sulfuric Acid (H₂SO₄).
- Ultrasonic probe.
- Filtration setup with 0.45 µm nylon membrane.
- Ultrapure water.
Procedure:
- In a fume hood, slowly add a 1:3 (v/v) mixture of HNO₃ to H₂SO₃ in a suitable glass container under vigorous stirring to create the sulfonitric solution.
- Gradually add the RC powder to the acid mixture. Continue stirring for the desired reaction time (e.g., 2-4 hours).
- Carefully filter the resulting functionalized carbon (RCF) through a 0.45 µm nylon membrane.
- Wash the RCF cake repeatedly with ultrapure water until the filtrate reaches a neutral pH to ensure complete acid removal.
- Disperse the final RCF product in ultrapure water at a concentration of 1.0 mg mL⁻¹ using an ultrasonic probe for 2 sets of 10 minutes to create a homogeneous suspension.
Protocol: Construction of an AChE Biosensor with a Metal-Organic Framework (MnMOF) Platform
This protocol describes a gentle method for constructing an AChE-immobilized platform (AChE@MnMOF) that demonstrates superior stability and resistance to harsh environments compared to free enzyme. [13]
Principle: A metal-organic framework (MnMOF) synthesized from squaric acid and manganese ions under mild conditions provides a porous, high-surface-area carrier for AChE. The resulting platform protects the enzyme and can be integrated into an electrochemical sensor based on charge repulsion effects.
Materials:
- Acetylcholinesterase (AChE) from Electrophorus electricus (e.g., Type VI-S, lyophilized powder).
- Squaric acid, Sodium Hydroxide (NaOH), Manganese(II) chloride tetrahydrate (MnCl₂·4H₂O).
- Ethanol.
- Phosphate Buffered Saline (PBS, 0.1 M, pH 7.4).
- Fluorine-doped Tin Oxide (FTO) or Glassy Carbon Electrode (GCE).
Procedure:
- Synthesis of AChE@MnMOF: a. Prepare a 0.1 M squaric acid sodium solution by dissolving squaric acid in a 0.2 M NaOH solution with ultrasonication. b. Add 1 mg of AChE to 0.5 mL of the prepared squaric acid sodium solution. c. In a separate vial, prepare a 0.1 M solution of MnCl₂·4H₂O in a 15% ethanol solution. d. Mix the two solutions and allow the reaction to proceed at room temperature for a defined period (e.g., 2 hours). The resulting composite is AChE@MnMOF.
- Electrode Modification and Biosensor Assembly: a. Polish the bare GCE (or clean the FTO electrode) following standard electrochemical preparation procedures. b. Deposit a precise aliquot (e.g., 5-10 µL) of the AChE@MnMOF suspension onto the electrode surface. c. Allow the modified electrode to dry at room temperature, forming a stable film.
Diagram: Experimental Workflow for AChE Biosensor Construction
The Scientist's Toolkit: Essential Research Reagents
The following table lists key materials used in the development of high-performance AChE biosensors, as cited in recent literature.
Table 2: Essential Reagents for AChE Biosensor Development
| Material / Reagent | Function / Rationale | Example Application |
|---|---|---|
| Renewable Carbon (RC) [23] | Sustainable, carbon-rich platform derived from biomass; moderate surface area and surface functional groups enable effective enzyme binding. | Base material for functionalization and AChE immobilization in pesticide detection. |
| SnO₂ Nanoparticles (NPs) [21] | n-type semiconductor; provides high surface area, good biocompatibility, and strong electrocatalytic activity. | Component of nanocomposite (with carboxylic graphene) for enhanced electron transfer. |
| Carboxylic Graphene (CGR) [21] | Graphene oxide with -COOH groups; offers large surface area, excellent conductivity, and functional groups for covalent enzyme attachment. | Used with SnO₂ NPs and Nafion to form a highly conductive nanocomposite film. |
| Nafion (NF) [21] | Perfluorosulfonated ionomer; acts as a protective membrane, provides chemical inertness, and helps stabilize the modified electrode surface. | Protective membrane and dispersion matrix for nanocomposites on the electrode. |
| Squaric Acid [13] | Organic ligand for MOF synthesis; allows for construction of the immobilization platform under mild, enzyme-friendly conditions. | Ligand for constructing the MnMOF enzyme carrier (AChE@MnMOF). |
| Manganese Ions (Mn²⁺) [13] | Metal center for MOF coordination; forms stable structures with squaric acid, creating a porous support for AChE. | Metal center for the MnMOF in the AChE immobilization platform. |
| Chitosan (CS) [21] | Natural biopolymer; used as a hydrogel for enzyme encapsulation, provides biocompatibility and enhances electron shuttling. | Hydrogel matrix for entrapping AChE on the surface of modified electrodes. |
The strategic selection and optimization of support materials based on biocompatibility, surface area, and functionalization potential are paramount for pushing the boundaries of AChE-based biosensors. The protocols and data presented herein provide a concrete foundation for researchers to engineer advanced immobilization platforms. By meticulously controlling these material properties, scientists can develop next-generation biosensors with enhanced sensitivity, stability, and application range, from environmental pesticide monitoring to neurodegenerative disease research.
Cutting-Edge Immobilization Methodologies and Their Real-World Applications
Physical Adsorption and Entrapment on Porous Silicon and other Nanomatrices
The immobilization of enzymes, such as acetylcholinesterase (AChE), is a critical technique for enhancing the stability and reusability of biocatalysts in industrial processes and biosensing applications [24]. Among the various support matrices available, porous silicon (PSi) and electrospun nanofibers stand out due to their high surface area-to-volume ratio, tunable porosity, and biocompatibility [24] [25]. PSi, in particular, with its interconnected pore network and easily modifiable surface chemistry, is ideally suited for enzyme anchoring, leading to improved operational stability and catalytic performance [26]. These immobilization strategies are pivotal in the development of robust biosensors and biocatalytic systems, especially in the context of drug development where AChE is a key target.
This document provides detailed application notes and protocols for the physical adsorption and entrapment of AChE on porous silicon and within electrospun nanofibers, framed within a broader thesis on AChE immobilization techniques for electrodes.
Acetylcholinesterase Immobilization on Porous Silicon
Physical Adsorption on Porous Silicon
Principle: Physical adsorption relies on non-covalent interactions (e.g., van der Waals forces, hydrophobic interactions, hydrogen bonding) between the enzyme and the porous silicon surface. This method is simple and preserves enzyme activity by avoiding harsh chemical reactions [24].
Protocol:
- PSi Substrate Preparation: Fabricate a mesoporous silicon layer (average pore size below 15 nm) on a p-type silicon wafer via electrochemical anodization in an HF-based electrolyte [26].
- Surface Activation: Treat the freshly etched, hydrogen-terminated PSi surface with a mild oxidizing agent or piranha solution to create a hydrophilic, hydroxyl-rich surface, which enhances enzyme binding [26].
- Enzyme Immobilization: Incubate the activated PSi substrate in a phosphate buffer (pH 7-8) containing AChE (e.g., from Electrophorus electricus) for a defined period (e.g., 1-2 hours) at room temperature [24].
- Washing: Gently rinse the PSi-AChE conjugate with buffer to remove any loosely bound enzyme.
Performance Data of Adsorbed AChE: Table 1: Characteristic performance data for AChE immobilized on PSi via physical adsorption.
| Parameter | Performance | Experimental Conditions |
|---|---|---|
| Activity Retention | ~50% retained activity | After immobilization, assessed via Ellman's assay [24] |
| Thermal Stability | Stable up to 90°C | Retained activity after exposure to high temperature [24] |
| pH Stability | Broad range (4–9) | Retained activity across pH values [24] |
| Reusability | Up to 3 cycles | Enzyme activity retained over repeated use [24] |
| Shelf Life | 44 days | Storage stability at 4°C [24] |
Covalent Immobilization on Modified Porous Silicon
Principle: Covalent attachment involves the formation of stable bonds (e.g., amide bonds) between functional groups on the enzyme and a chemically modified PSi surface. This method typically provides higher stability and prevents enzyme leaching [26].
Two primary strategies for covalent attachment are highlighted below:
Figure 1: Two chemical pathways for covalent immobilization of AChE on a porous silicon surface.
Protocol for Path A (Hydrosilylation and Amine Coupling):
- Hydrosilylation: React the hydrogen-terminated PSi surface with undecylenic acid (an ω-alkenoic acid) under inert atmosphere to form a stable carboxyl-terminated (PSi-COOH) monolayer via Si-C bonds [26].
- Activation: Incubate the PSi-COOH surface with a mixture of N-hydroxysuccinimide (NHS) and N-ethyl-N'-(3-dimethylaminopropyl)-carbodiimide (EDC) in water to activate the carboxyl groups, forming reactive succinimidyl esters (PSi-COOSuc) [26].
- Enzyme Coupling: Expose the activated surface to a solution of AChE. The primary amine groups on the enzyme's lysine residues will react with the esters to form stable amide bonds [26].
- Washing: Rinse thoroughly with buffer to remove non-covalently bound enzyme.
Protocol for Path B (Silanization and Carboxyl Coupling):
- Hydroxylation: Oxidize the hydrogen-terminated PSi in a piranha solution to create a homogeneous hydroxyl-terminated (Si-OH) surface [26].
- Silanization: React the hydroxylated PSi with 3-aminopropyltriethoxysilane (APTES) to form an amine-terminated (PSi-NH₂) surface via siloxane (Si-O-Si) linkages [26].
- Enzyme Coupling: Incubate the PSi-NH₂ surface with AChE in the presence of NHS/EDC. The carbodiimide chemistry activates the carboxylic acid groups on the enzyme (e.g., from aspartic or glutamic acid residues), which then form amide bonds with the surface amine groups [26].
- Washing: Rinse thoroughly with buffer.
Characterization: Success of surface modification and enzyme immobilization can be confirmed by Fourier Transform Infrared Spectroscopy (FTIR), which detects characteristic bonds (e.g., amide I and II bands), and by contact angle measurements, which reveal changes in surface wettability and can infer enzyme orientation [26].
Enzyme Entrapment in Electrospun Nanofibers
Principle: Enzyme entrapment and encapsulation involve incorporating the enzyme within a three-dimensional polymer network during the electrospinning process. This method protects the enzyme from harsh environmental conditions and minimizes leaching [25].
Figure 2: Workflow for encapsulating enzymes within electrospun nanofibers.
Protocol:
- Polymer Solution Preparation: Dissolve a suitable polymer (e.g., poly(methyl methacrylate) - PMMA, sodium alginate, poly(vinyl chloride)) in an appropriate organic solvent [25].
- Enzyme-Polymer Mixture: Gently mix the AChE enzyme solution into the polymer solution to achieve a homogeneous dispersion, avoiding conditions that could denature the enzyme.
- Electrospinning: Load the mixture into a syringe. Use a syringe pump to feed the solution through a metallic needle charged with a high voltage (typically 10-25 kV). The charged jet is drawn toward a grounded collector, solidifying into continuous nanofibers with the enzyme encapsulated within [25].
- Storage: Store the resulting nanofiber mat in a dry and cool environment.
Performance Advantages: Studies with other enzymes, like laccase, demonstrate the benefits of encapsulation. Laccase encapsulated in PMMA/Fe₃O₄ nanofibers achieved 100% immobilization yield and retained 90% of its initial activity after 40 days of storage, outperforming covalently bound enzymes [25]. Similarly, horseradish peroxidase (HRP) encapsulated in sodium alginate/poly(vinyl chloride) nanofibers was highly effective, degrading over 80% of specific pharmaceuticals in wastewater within 24 hours [25].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key reagents, materials, and equipment used in AChE immobilization protocols.
| Item | Function / Role | Example Use Case |
|---|---|---|
| Porous Silicon (PSi) | High-surface-area support matrix | Substrate for physical adsorption and covalent attachment of AChE [24] [26]. |
| Acetylcholinesterase (AChE) | Target enzyme for immobilization | Biocatalyst for hydrolysis of acetylthiocholine; model for inhibitor studies [24] [26]. |
| Undecylenic Acid | Provides surface carboxyl groups | Used in hydrosilylation for covalent immobilization path A [26]. |
| 3-Aminopropyltriethoxysilane (APTES) | Provides surface amine groups | Used in silanization for covalent immobilization path B [26]. |
| NHS & EDC | Crosslinking/coupling agents | Activates carboxyl groups for amide bond formation with enzyme amines [26]. |
| Electrospinning Polymers (e.g., PMMA) | Nanofiber matrix for entrapment | Forms the protective scaffold for enzyme encapsulation [25]. |
| Acetylthiocholine Iodide | Enzyme substrate | Used in Ellman's assay to measure AChE activity [24]. |
| 5,5'-Dithiobis-(2-nitrobenzoic acid) (DTNB) | Chromogenic agent (Ellman's reagent) | Produces a yellow-colored product upon reaction with thiocholine, allowing spectrophotometric activity measurement [26]. |
Comparative Analysis and Application Notes
Selection Guide:
- Physical Adsorption: Best for rapid prototyping and applications where minimal surface modification is desired. Its main drawbacks are potential enzyme leaching and lower stability under harsh conditions [24].
- Covalent Attachment: Ideal for applications requiring high operational stability and reusability, such as reusable biosensor electrodes or flow-through bioreactors. The chemical modification process is more complex and requires careful optimization to maintain enzyme activity [26].
- Entrapment/Encapsulation: Superior for protecting enzymes from denaturation and for use in harsh environments (e.g., organic solvents, extreme pH). It is well-suited for wastewater treatment and controlled release systems [25].
Activity Assay Protocol (Ellman's Method): The activity of free and immobilized AChE can be quantified using Ellman's assay [24] [26].
- Prepare a reaction mixture containing acetylthiocholine iodide (substrate) and DTNB (Ellman's reagent) in phosphate buffer (pH ~8.0).
- Add the AChE sample (free enzyme or immobilized substrate).
- Immediately monitor the increase in absorbance at 412 nm over time using a spectrophotometer. The rate of increase is proportional to the enzyme activity, as AChE hydrolyzes acetylthiocholine to release thiocholine, which reacts with DTNB to form 2-nitro-5-thiobenzoate (yellow).
Conclusion: The choice of immobilization technique—physical adsorption on porous silicon, covalent attachment on functionalized PSi, or entrapment within electrospun nanofibers—depends on the specific application requirements for stability, reusability, and activity retention. Porous silicon offers a versatile and high-capacity platform, particularly for biosensing applications within electrode design. In contrast, electrospun nanofibers provide a robust protective environment for enzymes in challenging process conditions. These advanced immobilization strategies significantly enhance the practicality of AChE and other enzymes in industrial biocatalysis, pharmaceutical screening, and environmental monitoring.
Covalent Immobilization on Functionalized Electrodes and Nanoparticle Composites
Application Note: Advanced Immobilization Platforms for Enhanced Biosensing
This application note details specialized protocols for covalent immobilization of acetylcholinesterase (AChE) on functionalized electrodes and nanoparticle composites, supporting ongoing thesis research on biosensor development for environmental and pharmaceutical applications. Covalent immobilization provides superior enzyme stability, prevents enzyme leakage, and maintains bioactivity under operational conditions, making it essential for reliable biosensor performance [27]. These techniques enable the creation of robust sensing platforms for detecting organophosphorus pesticides, carbamate insecticides, pharmaceutical agents, and heavy metals through AChE inhibition mechanisms [13] [28] [29].
The protocols described herein leverage nanomaterial-enhanced surfaces to address critical challenges in biosensor fabrication, including inefficient electron transfer, enzyme denaturation on metal surfaces, and limited operational stability [30] [31]. By creating stable covalent linkages between enzyme functional groups and activated support surfaces, these methods significantly improve biosensor lifespan while maintaining high catalytic efficiency.
Comparative Performance of Immobilization Platforms
Table 1: Analytical performance of AChE biosensors using different covalent immobilization strategies
| Immobilization Platform | Target Analyte | Linear Range | Detection Limit | Stability/Reusability | Reference |
|---|---|---|---|---|---|
| MnMOF-squaric acid | Chlorazophos | Not specified | 0.532 ng/mL | Enhanced stability vs. free AChE; resistant to harsh conditions | [13] |
| Electrodeposited Au nanoparticles | Carbofuran | Not specified | nM concentrations | Stable response; reusable | [31] |
| Screen-printed carbon electrode (direct covalent) | Arsenic(III) | 1×10⁻⁸ to 1×10⁻⁷ M | 1.1×10⁻⁸ M | RSD <4%; good reproducibility | [28] |
| 3D-printed flow-through reactor | Carbofuran | 10 nM - 0.1 μM | 10 nM | Easy mounting; low-cost replaceable parts | [29] |
| 3D-printed flow-through reactor | Donepezil | 1.0 nM - 1.0 μM | 1.0 nM | Suitable for point-of-care testing | [29] |
Table 2: Advantages and limitations of different covalent immobilization approaches
| Immobilization Method | Advantages | Limitations | Optimal Applications |
|---|---|---|---|
| Metal-Organic Frameworks (MOFs) | High surface area, tunable porosity, protective microenvironment | Strict synthesis conditions, potential coordination bond disruption | Sensitive detection systems requiring enzyme protection |
| Gold Nanoparticles | Enhanced electron transfer, roughened surface for better enzyme attachment | Higher cost, complex characterization | Fundamental studies on electron transfer mechanisms |
| Screen-printed Electrodes | Disposable use, cost-effective, reproducible mass production | Limited surface area, lower enzyme loading | Field-deployable sensors, single-use applications |
| Flow-through Reactors | Continuous operation, sample processing capability | More complex instrumentation, potential for clogging | Automated screening systems, continuous monitoring |
Protocol 1: AChE Immobilization on MnMOF Platform for Charge Repulsion-Based Detection
Principle
This protocol describes the covalent immobilization of AChE on squaric acid-based manganese metal-organic frameworks (MnMOF) for electrochemical detection of organophosphorus pesticides (OPs) based on charge repulsion effects [13]. The positively charged thiocholine (TCh) produced from acetylthiocholine hydrolysis creates charge repulsion with an equally positively charged electrochemical probe (Ru[(NH₃)₆]³⁺), reducing the electrochemical signal. OPs inhibit AChE activity, reducing TCh production and consequently weakening the repulsion effect, thereby restoring electrical signals in a concentration-dependent manner.
Materials and Reagents
Table 3: Essential research reagents for MnMOF immobilization protocol
| Reagent/Material | Specifications | Function in Protocol |
|---|---|---|
| Acetylcholinesterase (AChE) | Electric eel, Type III, ≥1000 U/mg | Biological recognition element for pesticide detection |
| Squaric acid (Quadratic acid) | 0.1 M in 0.2 M NaOH | Organic ligand for MOF construction |
| Manganese chloride tetrahydrate | 0.1 M in 15% ethanol | Metal ion source for MOF coordination |
| Acetylthiocholine (ATCh) | Substrate for enzymatic reaction | Enzyme substrate producing electroactive thiocholine |
| Ru[(NH₃)₆]³⁺ | Electrochemical probe | Positively charged mediator for charge repulsion detection |
| Fluorine-doped Tin Oxide (FTO) electrodes | Conducting transparent electrodes | Electrode substrate for biosensor assembly |
| Phosphate buffer | 0.1 M, pH 7.4 | Optimal pH environment for enzyme activity |
Step-by-Step Procedure
Synthesis of AChE@MnMOF
- Ligand Solution Preparation: Dissolve 0.1 M squaric acid in 0.2 M NaOH solution using ultrasonication for 5 minutes until completely dissolved.
- Enzyme Addition: Add 1 mg AChE to 0.5 mL of the prepared squaric acid sodium solution. Mix gently by swirling to avoid enzyme denaturation.
- Metal Solution Preparation: Dissolve 0.1 M manganese chloride tetrahydrate in 15% ethanol solution.
- Mixing and Reaction: Combine the enzyme-ligand solution with 0.5 mL manganese chloride solution. Allow the mixture to react at room temperature for 2 hours without disturbance.
- Collection and Washing: Centrifuge the resulting AChE@MnMOF composite at 5000 rpm for 10 minutes. Discard supernatant and resuspend in 0.1 M phosphate buffer (pH 7.4). Repeat washing twice.
Electrode Modification and Biosensor Assembly
- FTO Cleaning: Clean FTO electrodes sequentially with acetone, ethanol, and deionized water using ultrasonication for 10 minutes each.
- Composite Deposition: Drop-cast 10 μL of AChE@MnMOF suspension onto the pre-cleaned FTO electrode surface.
- Drying: Allow the modified electrode to dry overnight at 4°C under controlled humidity.
- Storage: Store the prepared biosensor at 4°C in dry conditions when not in use.
Inhibition Assay for Pesticide Detection
- Baseline Measurement: Record the amperometric response in 0.1 M phosphate buffer (pH 7.4) containing 0.3 mM ATCh and 50 μM Ru[(NH₃)₆]³⁺ at applied potential of -0.25 V vs. Ag/AgCl.
- Inhibition Step: Immerse the biosensor in sample containing target OP pesticide for 15 minutes.
- Signal Measurement: Re-measure the amperometric response under identical conditions as baseline.
- Quantification: Calculate inhibition percentage using the formula: % Inhibition = [(I₀ - I)/I₀] × 100, where I₀ and I are currents before and after inhibition.
Critical Parameters and Optimization
- Enzyme Loading: 1 mg AChE per mL of ligand solution provides optimal activity retention while preventing overcrowding.
- pH Optimization: pH 7.4 phosphate buffer maintains enzyme stability and activity.
- Inhibition Time: 15-minute inhibition provides sufficient sensitivity while maintaining practical analysis time.
- Temperature Control: All procedures should be performed at 4°C when handling enzyme solutions to prevent denaturation.
Protocol 2: Electrodeposited Gold Nanoparticle Platform for Enhanced AChE Stabilization
Principle
This protocol utilizes electrodeposited gold nanoparticles (AuNPs) to create a roughened electrode surface that enhances AChE immobilization and stabilizes enzyme activity [31]. The nanostructured surface increases effective surface area and provides a favorable microenvironment for enzyme orientation, significantly improving electron transfer efficiency compared to planar gold electrodes. The immobilized AChE catalyzes acetylthiocholine hydrolysis to thiocholine, which is electrochemically oxidized at the electrode surface, enabling detection of AChE inhibitors through signal reduction.
Materials and Reagents
Table 4: Essential research reagents for AuNP immobilization protocol
| Reagent/Material | Specifications | Function in Protocol |
|---|---|---|
| Hydrogen tetrachloroaurate | 99.99% purity | Gold precursor for nanoparticle synthesis |
| Gold wire electrode | 99.99% purity, 0.2 mm diameter | Working electrode substrate |
| Phosphate buffer saline | 0.1 M, pH 7.4 | Electrochemical measurement buffer |
| Acetylthiocholine chloride | ≥99% purity | Enzyme substrate |
| Aluminum oxide sanding gel | 0.3 μm and 0.05 μm | Electrode polishing material |
| Potassium chloride | Analytical grade | Supporting electrolyte |
Step-by-Step Procedure
Electrode Pretreatment
- Mechanical Polishing: Polish gold wire electrodes sequentially with 0.3 μm and 0.05 μm aluminum oxide sanding gel to create a mirror-finish surface.
- Electrochemical Cleaning: Perform cyclic voltammetry in 0.5 M H₂SO₄ from -0.2 to +1.5 V (vs. Ag/AgCl) at 100 mV/s until stable voltammogram is obtained.
- Rinsing: Rinse thoroughly with deionized water between each polishing step and after electrochemical cleaning.
Gold Nanoparticle Electrodeposition
- Solution Preparation: Prepare electrodeposition solution containing 0.5 mM HAuCl₄ in 0.1 M KCl.
- Electrodeposition: Apply constant potential of -0.2 V (vs. Ag/AgCl) for 300 seconds to the pre-cleaned gold electrode in the deposition solution under gentle stirring.
- Characterization: Monitor the deposition process by observing the color change from metallic gold to dull reddish color, indicating successful nanoparticle formation.
- Rinsing and Drying: Rinse the AuNP-modified electrode with deionized water and dry under nitrogen stream.
AChE Immobilization
- Enzyme Adsorption: Incubate the AuNP-modified electrode in AChE solution (0.1 mg/mL in pH 7.4 phosphate buffer) for 12 hours at 4°C.
- Washing: Gently rinse with cold phosphate buffer to remove loosely adsorbed enzyme.
- Storage: Store the prepared biosensor at 4°C in phosphate buffer when not in use.
Amperometric Measurement
- Experimental Setup: Use conventional three-electrode system with AChE-AuNP electrode as working electrode, Ag/AgCl reference electrode, and platinum counter electrode.
- Measurement Conditions: Apply potential of +0.6 V (vs. Ag/AgCl) in stirring phosphate buffer solution (pH 7.4).
- Substrate Addition: Add acetylthiocholine aliquots to desired concentration (typically 0.1-0.5 mM final concentration).
- Signal Recording: Record steady-state oxidation current (typically within 30-60 seconds).
Critical Parameters and Optimization
- Electrodeposition Potential: -0.2 V provides optimal nanoparticle size distribution and surface coverage.
- Enzyme Concentration: 0.1 mg/mL AChE provides monolayer coverage without excessive multilayer formation.
- Adsorption Time: 12-hour incubation ensures sufficient enzyme loading while maintaining activity.
- Applied Potential: +0.6 V enables thiocholine oxidation while minimizing interfering reactions.
Troubleshooting and Technical Considerations
Common Implementation Challenges
- Low Enzyme Activity Retention: Ensure proper pH control during immobilization (pH 7.0-7.5) and avoid dehydration of enzyme layer. For MOF-based platforms, verify that metal ions do not denature the enzyme by testing different metal-to-ligand ratios [13].
- High Background Current: Implement additional blocking steps with ethanolamine or bovine serum albumin (BSA) after enzyme immobilization to cover unreacted functional groups [27].
- Poor Reproducibility: Standardize electrode pretreatment procedures precisely and control nanoparticle deposition parameters rigorously. For AuNP platforms, ensure consistent electrodeposition time and potential [31].
- Signal Drift: Allow sufficient stabilization time after electrode modification (typically 24 hours at 4°C) before initial use. For flow-through systems, ensure stable flow rates and temperature control [29].
Validation Methods
- Activity Assay: Verify immobilized enzyme activity by comparing thiocholine production rates between free and immobilized AChE using Ellman's assay.
- Surface Characterization: Employ atomic force microscopy (AFM) to verify nanoparticle deposition and surface roughness [31].
- Electrochemical Characterization: Use cyclic voltammetry and electrochemical impedance spectroscopy to confirm successful enzyme immobilization and assess electron transfer efficiency.
- Stability Testing: Evaluate operational stability through repeated measurements over extended time periods and thermal stability by testing activity retention at different temperatures.
These application notes and protocols provide detailed methodologies for covalent immobilization of AChE on functionalized electrodes and nanoparticle composites, supporting advanced biosensor development for thesis research. The MnMOF platform offers exceptional enzyme stabilization against temperature and organic solvent denaturation, while the AuNP-enhanced electrode significantly improves electron transfer efficiency. Both platforms enable sensitive detection of AChE inhibitors with detection limits reaching nanomolar concentrations, making them suitable for environmental monitoring, food safety testing, and pharmaceutical applications.
The covalent immobilization approaches described herein provide superior performance compared to physical adsorption methods, particularly in terms of operational stability, reusability, and resistance to environmental perturbations. Researchers can select the appropriate platform based on specific application requirements, with MnMOF composites offering enhanced stability in harsh conditions and AuNP-modified electrodes providing superior electrochemical characteristics for sensitive amperometric detection.
Metal-Organic Frameworks (MOFs) as Advanced Enzyme Stabilization Platforms
Metal-organic frameworks (MOFs) have emerged as transformative materials for enzyme immobilization, addressing critical challenges in biocatalysis and biosensing. These porous crystalline structures, formed through coordination bonds between metal ions and organic ligands, provide an ideal microenvironment for stabilizing enzymes like acetylcholinesterase (AChE) while maintaining their catalytic efficiency [32] [33]. Their structural versatility, tunable porosity, and exceptional surface areas offer significant advantages over traditional immobilization supports, enabling breakthroughs in biosensor design, particularly for detecting environmental contaminants such as organophosphorus pesticides (OPs) [13] [18].
The integration of MOF technology with enzyme-based electrodes represents a paradigm shift in biosensing capabilities, overcoming inherent limitations of free enzymes including structural instability, sensitivity to environmental conditions, and poor reusability [32]. By providing protective nano-confinement and enhanced mass transfer pathways, MOF-based immobilization platforms significantly improve enzyme stability under operational conditions while enabling sophisticated sensing architectures for food safety monitoring, medical diagnostics, and environmental protection [32] [33].
MOF-Based Enzyme Immobilization Strategies
Fundamental Immobilization Approaches
The strategic immobilization of enzymes within MOF structures relies on precisely engineered interactions between the enzyme molecules and the porous framework. Researchers have developed multiple technical approaches to achieve optimal enzyme loading, activity retention, and stability enhancement, each with distinct advantages for specific applications.
Table 1: Comparison of MOF-Based Enzyme Immobilization Strategies
| Immobilization Method | Key Mechanism | Advantages | Limitations | Suitable MOF Types |
|---|---|---|---|---|
| In Situ Encapsulation | Co-precipitation or co-crystallization during MOF synthesis [34] [35] | High enzyme loading; minimal enzyme leaching; maximum protection | Harsh synthesis conditions may denature enzymes; limited control over MOF morphology | ZIF-8, ZIF-90, HKUST-1 [34] |
| Surface Immobilization | Covalent bonding or physical adsorption to pre-formed MOFs [35] | Preserves MOF crystallinity; simple procedure; suitable for large enzymes | Lower enzyme loading; potential enzyme leaching; limited protection | UIO-66-NH₂, MIL-101-NH₂ [35] |
| Pore Diffusion | Enzyme infiltration into pre-synthesized MOF pores [35] | Maintains enzyme native structure; mild conditions | Limited to enzymes smaller than MOF pores; potential leaching | Large-pore MOFs (MIL-100, MIL-101) [35] |
| Core-Shell Sequential Encapsulation | Controlled positioning of multiple enzymes through sequential addition [34] | Optimal spatial arrangement for cascade reactions; enhanced mass transfer | Complex synthesis optimization; requires compatibility between enzymes | ZIF-8, ZIF-90 [34] |
Advanced Design Considerations
Recent innovations in MOF-enzyme composite design have focused on overcoming the fundamental trade-offs between framework stability and enzyme activity. While MOF stability often requires harsh synthesis conditions, enzyme activity preservation necessitates mild environments [36]. Advanced strategies including defect engineering, biomimetic mineralization, and hierarchical pore architecture have successfully addressed these challenges, creating optimized microenvironments that maintain enzymatic conformation and catalytic efficiency [32] [37].
For AChE immobilization specifically, the selection of MOF composition and immobilization method must consider the enzyme's molecular dimensions, surface charge distribution, and sensitivity to organic solvents. The use of squaric acid-based MOFs synthesized under mild conditions has demonstrated exceptional success in maintaining AChE activity while providing enhanced stability against thermal denaturation and organic solvent exposure [13].
Application Notes: AChE-MOF Biosensors for Pesticide Detection
Operational Principles and Sensing Mechanisms
AChE-MOF biosensors for organophosphorus pesticide detection operate primarily through enzyme inhibition principles, where OPs irreversibly bind to the AChE active site, reducing catalytic activity proportionally to pesticide concentration [13] [18]. The detection methodology involves monitoring the enzymatic hydrolysis of acetylthiocholine iodide to thiocholine and acetic acid, with the electroactive thiocholine product generating measurable signals at the electrode interface [13] [28].
Innovative sensing mechanisms have significantly enhanced detection sensitivity and specificity. The charge repulsion effect represents a particularly advanced approach, where positively charged thiocholine electrostatically repels an equally charged redox probe (Ru[(NH₃)₆]³⁺), thereby decreasing electrochemical signal. OP inhibition reduces thiocholine production, consequently weakening this repulsion effect and restoring electrical signals in a quantifiable manner [13].
Performance Metrics and Real-World Applications
AChE-MOF biosensors have demonstrated exceptional analytical performance in both controlled laboratory settings and complex real-world samples. The AChE@MnMOF platform achieved a detection limit of 0.532 ng/mL for chlorazophos with satisfactory recoveries (88.15–107.86%) in actual samples including pears, cabbages, and tap water [13]. These biosensors maintain operational stability under challenging environmental conditions and exhibit significantly extended shelf life compared to free enzyme systems.
The practical implementation of these biosensing platforms has been enhanced through integration with disposable screen-printed carbon electrodes (SPCEs), which offer important advantages for field deployment including low cost, minimal sample volume requirements, and elimination of memory effects between measurements [38] [28]. This combination of sophisticated MOF-enzyme biocomposites with economical electrode platforms creates an optimal balance between analytical performance and practical applicability for environmental monitoring and food safety assurance.
Experimental Protocols
Protocol 1: Synthesis of AChE@MnMOF Biocomposite
Principle: This protocol describes the one-pot synthesis of acetylcholinesterase immobilized on squaric acid-based manganese MOF (AChE@MnMOF) under mild conditions to preserve enzymatic activity while ensuring robust immobilization [13].
Materials:
- Acetylcholinesterase (AChE) from Electrophorus electricus
- Squaric acid (3,4-dihydroxy-3-cyclobutene-1,2-dione)
- Manganese(II) chloride tetrahydrate (MnCl₂·4H₂O)
- Sodium hydroxide (NaOH)
- Absolute ethanol
- Deionized water
Procedure:
- Prepare 0.1 M squaric acid sodium solution by adding squaric acid (0.1 M) to 0.2 M NaOH solution with ultrasonication for 5 minutes until complete dissolution.
- Add 1 mg AChE to 0.5 mL of the prepared squaric acid sodium solution. Mix gently by inversion to avoid enzyme denaturation.
- Prepare 0.1 M manganese chloride tetrahydrate solution in 15% ethanol (0.5 mL).
- Combine the two solutions in a 2 mL vial and mix by gentle vortexing for 30 seconds.
- Incubate the reaction mixture at room temperature (25°C) for 2 hours without agitation.
- Collect the resulting AChE@MnMOF crystals by centrifugation at 5,000 × g for 5 minutes.
- Wash twice with 50 mM phosphate buffer (pH 7.4) to remove unimmobilized enzyme.
- Resuspend the final biocomposite in 1 mL phosphate buffer and store at 4°C until use.
Validation Parameters:
- Enzyme loading efficiency: Quantify by measuring initial and supernatant protein content using Bradford assay [13].
- Enzymatic activity: Assess using Ellman's method with acetylthiocholine as substrate [13].
- Morphological characterization: Analyze by scanning electron microscopy to confirm MOF crystallinity [13].
Protocol 2: Fabrication of AChE@MnMOF-Modified Electrode for OP Detection
Principle: This protocol details the construction of an electrochemical biosensor by modifying a screen-printed carbon electrode (SPCE) with the AChE@MnMOF biocomposite for sensitive detection of organophosphorus pesticides based on charge repulsion effects [13].
Materials:
- Screen-printed carbon electrodes (SPCEs)
- AChE@MnMOF biocomposite (from Protocol 1)
- Chitosan solution (0.5% w/v in 1% acetic acid)
- Phosphate buffer (50 mM, pH 7.4)
- Acetylthiocholine iodide (ATCh)
- Hexaammineruthenium(III) chloride (Ru[(NH₃)₆]³⁺)
- Organophosphorus pesticide standards (chlorazophos, parathion, etc.)
Procedure:
- Pre-treat SPCEs by applying +1.5 V for 60 seconds in phosphate buffer to activate the carbon surface.
- Prepare electrode modification ink by mixing 10 μL AChE@MnMOF suspension with 5 μL chitosan solution (0.5% w/v).
- Deposit 5 μL of the modification ink onto the SPCE working electrode area.
- Dry the modified electrode under ambient conditions (25°C) for 45 minutes.
- Cross-link the biocomposite layer by exposing to glutaraldehyde vapor (25% solution) for 5 seconds.
- Rinse the fabricated biosensor with phosphate buffer to remove unreacted glutaraldehyde.
- For inhibition assays, incubate the biosensor in OP standard solutions for 10 minutes at 25°C.
- Perform electrochemical measurements in solution containing 0.2 mM ATCh and 0.1 mM Ru[(NH₃)₆]³⁺ in phosphate buffer.
Electrochemical Measurement Parameters:
- Technique: Amperometry or differential pulse voltammetry
- Applied potential: +0.1 V (vs. Ag/AgCl reference)
- Equilibrium time: 60 seconds before measurement
- Measurement duration: 120 seconds
Calibration:
- Record steady-state current (I₀) in absence of OPs
- Record steady-state current (I) after OP exposure
- Calculate inhibition percentage as: % Inhibition = [(I₀ - I)/I₀] × 100
Figure 1: Experimental workflow for AChE@MnMOF biosensor fabrication and application in OP detection
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Research Reagent Solutions for AChE-MOF Biosensor Development
| Reagent/Material | Function/Application | Specification Notes | Supplier Examples |
|---|---|---|---|
| ZIF-8 Precursors | Framework for enzyme encapsulation [37] [34] | Zn²⁺ ions with 2-methylimidazole; mild synthesis conditions (aqueous, room temperature) | Sigma-Aldrich, Thermo Fisher |
| Squaric Acid (3,4-dihydroxy-3-cyclobutene-1,2-dione) | Organic ligand for MnMOF synthesis [13] | High-purity (>98%); enables mild synthesis conditions (room temperature, aqueous) | TCI Chemicals, Alfa Aesar |
| Acetylcholinesterase (AChE) | Recognition/biosensing element for OPs [13] [18] | From Electrophorus electricus; specific activity >1000 U/mg; lyophilized powder | Sigma-Aldrich, Worthington Biochemical |
| Acetylthiocholine Iodide | Enzyme substrate for electrochemical detection [13] [28] | Electrochemically active product (thiocholine); >98% purity; light-sensitive storage | Sigma-Aldrich, Cayman Chemical |
| Hexaammineruthenium(III) Chloride | Electrochemical probe for charge repulsion sensing [13] | Positively charged redox mediator; >98% purity; [Ru(NH₃)₆]³⁺/²⁺ redox couple | Strem Chemicals, Sigma-Aldrich |
| Screen-Printed Carbon Electrodes (SPCEs) | Disposable sensing platform [38] [28] | Three-electrode system (WE: carbon, RE: Ag/AgCl, CE: carbon); low-cost, mass-producible | Metrohm DropSens, PalmSens |
| Chitosan | Biopolymer for electrode modification [13] | Natural polysaccharide; 0.5% w/v in 1% acetic acid; enhances biocomposite adhesion | Sigma-Aldrich, Primex |
Technological Outlook and Future Perspectives
The integration of MOF platforms with enzyme-based biosensing technologies continues to evolve toward increasingly sophisticated architectures. Future developments are anticipated in several key areas, including the creation of multi-enzyme cascade systems with spatially controlled positioning within core-shell MOF structures [34]. These advanced configurations optimize substrate channeling and reaction efficiency while providing enhanced protection against harsh environmental conditions.
Emerging research priorities include the development of biologically derived MOFs, magnetic hierarchical porous MOFs, and nanoMOFs designed specifically for biomedical applications [33]. Additionally, novel materials inspired by MOF technology such as covalent organic frameworks (COFs) and metal-organic aerogels (MOAs) offer complementary properties that may further expand the capabilities of enzyme immobilization platforms [33]. The convergence of these advanced materials with microfluidic pretreatment systems and intelligent sensing technologies will ultimately enable comprehensive "screening-confirmation" frameworks that combine rapid biosensing with confirmatory chromatographic techniques for enhanced reliability in environmental monitoring and food safety applications [18].
Gold Nanoparticle-Enhanced Electrodes for Improved Sensitivity and Stability
The immobilization of acetylcholinesterase (AChE) on electrode surfaces is a fundamental aspect of developing robust biosensors for neurotoxin detection, pharmaceutical screening, and environmental monitoring. AChE is a crucial enzyme that hydrolyzes the neurotransmitter acetylcholine and is a primary target for organophosphorus pesticides and nerve agents [31]. A significant challenge in this field is maintaining enzyme stability and catalytic efficiency upon immobilization on conventional electrode surfaces, which often leads to reduced sensor performance and lifespan [31] [13]. The integration of gold nanoparticles (AuNPs) has emerged as a transformative strategy to overcome these limitations. AuNPs create a high-surface-area, biocompatible interface that enhances enzyme loading, facilitates electron transfer, and stabilizes the immobilized enzyme structure [31] [39]. This application note details the quantitative performance benefits and provides standardized protocols for fabricating and characterizing AuNP-enhanced AChE biosensors, framed within ongoing thesis research on advanced enzyme immobilization techniques.
Performance Data and Comparative Analysis
The incorporation of AuNPs into AChE biosensors significantly enhances key performance metrics compared to conventional electrode designs. The following tables summarize quantitative improvements in sensitivity, stability, and overall detection capability.
Table 1: Comparative Sensor Performance with and without AuNP Enhancement
| Performance Parameter | Planar Gold Electrode (No AuNPs) | AuNP-Modified Electrode | Improvement Factor |
|---|---|---|---|
| Surface Roughness | Not Specified (Smooth) | ~67 nm [31] | N/A |
| Detection Limit for Carbofuran | Significantly Reduced Response [31] | Nanomolar (nM) concentrations [31] | >100x (estimated) |
| Enzyme Stability & Immobilization | Limited utility, reduced enzyme attachment [31] | Enhanced enzyme adsorption and stabilization [31] | Substantial |
| Electron Transfer Efficiency | Lower | Higher | Not Specified |
Table 2: Performance Comparison with Other Advanced AChE Immobilization Platforms
| Immobilization Platform | Target Analyte | Detection Limit | Key Advantage |
|---|---|---|---|
| AuNP-electrodeposited Electrode [31] | Carbofuran | nM range | Simple fabrication, enhanced sensitivity |
| AChE@MnMOF Platform [13] | Chlorazophos | 0.532 ng/mL | Superior storage stability & resistance to harsh environments |
| Organocatalytic Reaction (NNO) [40] | AChE Activity | 14.1 U L⁻¹ (LOD) | Eliminates need for secondary enzymes, real-time monitoring |
Experimental Protocols
Protocol 1: Electrodeposition of Gold Nanoparticles on a Planar Gold Electrode
This protocol creates a roughened, high-surface-area AuNP layer for enhanced AChE immobilization, adapted from foundational research [31].
Principle: Electrodeposition is used to deposit a layer of colloidal gold nanoparticles onto a planar gold electrode, creating a nanostructured surface that improves enzyme attachment and stability.
Materials:
- Planar gold working electrode (e.g., gold wire, 0.2 mm diameter)
- Hydrogen tetrachloroaurate (HAuCl₄) solution
- Potassium chloride (KCl) as a supporting electrolyte
- Aluminum oxide sanding gel for electrode polishing
- Deionized water
Procedure:
- Electrode Pretreatment: Polish the planar gold electrode thoroughly with aluminum oxide sanding gel. Rinse sequentially with deionized water and ethanol, then dry under a gentle stream of nitrogen gas.
- Electrodeposition Solution: Prepare a solution containing 0.5 mM HAuCl₄ and 0.1 M KCl.
- Nanoparticle Deposition: Immerse the cleaned gold electrode in the electrodeposition solution. Apply a constant potential of -0.2 V (vs. Ag/AgCl reference electrode) for 120 seconds under stirring conditions.
- Post-treatment: After deposition, gently rinse the modified electrode with deionized water to remove any loosely adsorbed ions or nanoparticles. Air-dry at room temperature.
- Verification: The successful deposition of AuNPs is indicated by a visual change from a metallic gold luster to a dull reddish color. Atomic Force Microscopy (AFM) can be used to confirm an increase in surface roughness (approximately 67 nm).
Protocol 2: Acetylcholinesterase Immobilization and Inhibition Assay for Pesticide Detection
This protocol describes the immobilization of AChE onto the AuNP-modified electrode and its application in sensing organophosphorus pesticides via enzyme inhibition [31].
Principle: The immobilized AChE catalyzes the hydrolysis of acetylthiocholine (ASCh) to produce thiocholine, which is electrochemically oxidized at the electrode surface. The presence of pesticides inhibits AChE, reducing the thiocholine signal, allowing for quantitative detection of the inhibitor.
Materials:
- AuNP-modified gold electrode (from Protocol 1)
- Acetylcholinesterase (AChE, Type III from electric eel)
- Acetylthiocholine chloride (ASCh)
- Organophosphate pesticide standard (e.g., carbofuran, malathion)
- Phosphate buffer saline (PBS, 0.1 M, pH 7.4)
Procedure:
- Enzyme Immobilization: Deposit 10 µL of an AChE solution (1 mg/mL in pH 7.4 buffer) onto the surface of the AuNP-modified electrode. Allow it to incubate for 60 minutes at 4°C for physical adsorption.
- Sensor Storage: After immobilization, store the AChE-modified electrode in a refrigerator at 4°C when not in use.
- Electrochemical Measurement:
- Place the AChE-AuNP electrode in an electrochemical cell containing 10 mL of stirred PBS.
- Add acetylthiocholine chloride to a final concentration of 0.5 mM.
- Apply an operating potential of +0.65 V (vs. Ag/AgCl) and record the amperometric current generated by the oxidation of thiocholine.
- Inhibition Assay (Pesticide Detection):
- Incubate the AChE-AuNP electrode in a sample solution containing the suspected pesticide inhibitor for 10 minutes.
- Rinse the electrode gently with PBS to remove unbound inhibitor.
- Measure the residual enzymatic activity by repeating Step 3 and recording the amperometric current.
- The percentage of inhibition is calculated as:
% Inhibition = [(I₀ - I₁) / I₀] × 100, where I₀ is the current before inhibition and I₁ is the current after inhibition.
Workflow and Signaling Pathways
The following diagrams illustrate the experimental workflow for biosensor fabrication and the signaling principle for pesticide detection.
Biosensor Fabrication and Application Workflow
Signaling Principle for AChE Inhibition Sensing
The Scientist's Toolkit: Research Reagent Solutions
Table 3: Essential Materials for AuNP-Enhanced AChE Biosensor Development
| Reagent/Material | Function/Role | Specific Example & Notes |
|---|---|---|
| Gold Electrode | Transducer base; platform for AuNP electrodeposition. | Planar gold wire (0.2 mm diameter); requires meticulous polishing before use [31]. |
| Hydrogen Tetrachloroaurate (HAuCl₄) | Gold precursor for nanoparticle electrodeposition. | Used at 0.5 mM concentration in KCl supporting electrolyte [31]. |
| Acetylcholinesterase (AChE) | Biological recognition element; catalyzes substrate hydrolysis. | Type III from electric eel; immobilized via physical adsorption on AuNP layer [31]. |
| Acetylthiocholine (ASCh) | Enzyme substrate; reaction product is electroactive. | Hydrolyzed to thiocholine and acetate; thiocholine oxidation generates sensing signal [31]. |
| Organophosphate Pesticide | Target analyte; inhibits AChE activity. | Carbofuran, malathion used as model inhibitors for biosensor validation [31] [41]. |
| Phosphate Buffered Saline (PBS) | Electrochemical cell medium; provides stable pH. | 0.1 M, pH 7.4; ensures optimal enzymatic activity during measurement [31]. |
Screen-Printed Carbon Electrodes (SPCEs) for Disposable and Point-of-Care Biosensors
Screen-printed carbon electrodes (SPCEs) represent a cornerstone technology in the development of disposable and point-of-care biosensors due to their cost-effectiveness, disposability, miniaturization potential, and simple electrode design [42]. These characteristics make them particularly valuable for applications ranging from environmental monitoring to clinical diagnostics. Within the broader context of acetylcholinesterase (AChE) immobilization techniques, SPCEs provide an excellent platform for creating sensitive biosensing systems for pesticide detection and other analytical applications [43]. The performance of these enzymatic biosensors depends critically on two key factors: the activity of the immobilized AChE and the electron transfer rate to the electrode [43]. Recent advances in surface modification techniques, including oxygen plasma treatment and nanocomposite development, have significantly enhanced SPCE performance, opening new avenues for robust biosensor development in point-of-care testing (POCT) scenarios [42].
Key Applications of SPCE-Based AChE Biosensors
SPCE-based biosensors incorporating immobilized acetylcholinesterase have found diverse applications, particularly in environmental monitoring and food safety. The inhibition of AChE by various pesticides forms the basis for highly sensitive detection systems.
- Pesticide Detection: AChE biosensors on SPCEs enable detection of carbamate and organophosphate pesticides. The enzyme catalyzes hydrolysis of acetylthiocholine to produce thiocholine, which is electrochemically oxidized. pesticide inhibitors reduce thiocholine production and oxidation current, allowing quantification [43].
- Food Safety Monitoring: Chemically modified SPCEs are invaluable for detecting pesticide residues, toxins, and other contaminants in food products, ensuring safety and quality [44].
- Point-of-Care Testing: The disposability and miniaturization of SPCEs make them ideal for rapid, on-site analysis in non-laboratory settings [42].
Performance Comparison of AChE Immobilization Techniques
The following table summarizes the performance characteristics of different AChE immobilization approaches on electrode surfaces, highlighting the impact of various modification strategies.
Table 1: Performance Characteristics of AChE-Based Biosensors with Different Electrode Modifications
| Electrode Modification | Immobilization Method | Target Analyte | Linear Range | Detection Limit | Stability/Reusability | Reference |
|---|---|---|---|---|---|---|
| PPy-IC-DS-AuNP Nanocomposite | Electrostatic adsorption | Carbaryl pesticide | 0.05 - 0.25 ng mL⁻¹ | 0.033 ng cm² mL⁻¹ | Good repeatability (RSD 1.8-3.7%); stable at -15°C [43] | [43] |
| Mesoporous Silicon | Physical adsorption | AChE inhibitors | Variable drug concentrations | Not specified | Reusable (3 cycles); 44-day shelf-life; pH (4-9) & thermal stability (up to 90°C) [45] | [45] |
| O₂ Plasma-treated SPCE | Covalent binding | Model analyte (via immunosensor) | Not specified | 0.50 ng/mL | N/A [42] | [42] |
| Bare SPCE (Control) | Physical adsorption | Model analyte (via immunosensor) | Not specified | 1.2 ng/mL | N/A [42] | [42] |
Experimental Protocols
Protocol: O₂ Plasma Surface Modification of SPCEs for Enhanced Biosensor Sensitivity
This protocol describes a method to functionalize the inert carbon surface of SPCEs, generating carboxyl groups that serve as scaffolds for covalent immobilization of biomolecules, thereby improving biosensor sensitivity [42].
Primary Materials:
- Screen-printed carbon electrodes (SPCEs)
- Oxygen plasma cleaner/chamber
- Antibody or enzyme for immobilization
- Coupling agents (e.g., EDC/NHS for covalent bonding)
Procedure:
- SPCE Preparation: Place clean, dry SPCEs into the plasma chamber.
- Plasma Treatment: Expose the SPCEs to oxygen plasma. The specific parameters (power, pressure, treatment time) must be optimized for the specific equipment and desired surface density of carboxyl groups.
- Post-Treatment Handling: Remove the modified SPCEs from the chamber. The surfaces now contain carboxyl groups and are ready for immediate functionalization.
- Biomolecule Immobilization:
- For covalent binding: Activate the surface carboxyl groups using a crosslinker like EDC/sulfo-NHS. Then, incubate with the antibody or enzyme solution [42].
- For physical adsorption: Simply incubate the plasma-treated SPCE with the biomolecule solution. Note that plasma treatment enhances physical adsorption by modifying surface chemistry and increasing available sites [42].
- Washing and Storage: Rinse the modified electrodes thoroughly with a suitable buffer to remove unbound molecules. Store in appropriate conditions until use.
Expected Outcomes: O₂ plasma treatment produces carboxyl groups on the electrode surface, altering electrochemical properties via electrostatic interactions. This treatment increases the number of antibody/enzyme adsorption sites, leading to a lower limit of detection (LOD) and higher sensitivity compared to bare SPCEs [42].
Protocol: Acetylcholinesterase Immobilization on Polypyrrole Nanocomposite for Carbaryl Detection
This protocol outlines the preparation of a nanocomposite-modified electrode for efficient AChE immobilization, resulting in a biosensor with high sensitivity for the pesticide carbaryl [43].
Primary Materials:
- Pyrrole monomer (double-distilled)
- Indigo carmine (IC), sodium dodecyl sulfate (DS)
- Tetrachloroaurate trihydrate (for AuNP synthesis)
- Acetylcholinesterase (AChE) from Electrophorus electricus
- Acetylthiocholine chloride (ATCl) substrate
- Carbaryl pesticide standard
Procedure:
- Nanocomposite Electrode Fabrication:
- Electropolymerize pyrrole in the presence of IC, DS, and pre-synthesized gold nanoparticles (AuNPs) to form a PPy-IC-DS-AuNP nanocomposite film on the electrode surface [43].
- The competition between DS and pyrrole during polymerization, especially above the critical micelle concentration (CMC) of DS, creates a surface with optimized conditions for enzyme binding [43].
- AChE Immobilization:
- Immobilize AChE onto the positively charged, hydrophilic surface of the PPy-IC-DS-AuNP nanocomposite via electrostatic interactions and physical adsorption [43].
- This interaction orients the enzyme's active site close to the electrode interface, promoting fast direct electron transfer.
- Biosensor Assay:
- Incubate the AChE-modified biosensor with a sample solution containing the pesticide carbaryl.
- Add the substrate acetylthiocholine chloride (ATCl). The production of electroactive thiocholine (TCh) is measured amperometrically.
- The inhibition of AChE by carbaryl causes a decrease in the TCh oxidation current, which is proportional to the carbaryl concentration [43].
- Nanocomposite Electrode Fabrication:
Expected Outcomes: This strategy allows for the immobilization of a high density of active enzymes. The biosensor exhibits a low detection limit (0.033 ng cm² mL⁻¹), high sensitivity, and good reproducibility for carbaryl [43].
Experimental Workflow and Signaling Visualization
AChE Biosensor Fabrication and Operation
This workflow illustrates the two main paths for biosensor operation: measuring normal enzyme activity (high signal) and measuring inhibited activity after pesticide exposure (low signal). The reduction in signal is proportional to the pesticide concentration [43].
AChE Inhibition Signaling Pathway
This diagram shows the core principle of AChE-based biosensors. In the normal catalytic cycle, AChE produces an electroactive product (TCh) that generates a measurable current. Pesticides inhibit AChE, preventing TCh production and reducing the electrochemical signal, enabling pesticide quantification [43].
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 2: Key Research Reagents and Materials for SPCE-based AChE Biosensors
| Item Name | Function/Description | Example Use Case |
|---|---|---|
| Screen-Printed Carbon Electrodes (SPCEs) | Disposable, cost-effective platform with working, counter, and reference electrodes integrated [42] [44]. | The foundational substrate for all biosensor constructions. |
| Acetylcholinesterase (AChE) | Enzyme that catalyzes acetylthiocholine hydrolysis; the biorecognition element for pesticides [45] [43]. | Immobilized on SPCE surface to create the sensing interface. |
| Acetylthiocholine Iodide/Chloride (ATCl) | Enzyme substrate; hydrolysis produces electroactive thiocholine [45] [43]. | Used in the electrochemical assay to measure enzyme activity. |
| Oxygen Plasma System | Surface modification tool that generates carboxyl groups on stable carbon surfaces [42]. | Functionalizes SPCEs for improved biomolecule immobilization (covalent or adsorbed). |
| Polypyrrole (PPy) & Dopants (IC, DS) | Conducting polymer matrix for nanocomposites; enhances surface area, conductivity, and enzyme loading [43]. | Creates a favorable microenvironment on SPCEs for AChE immobilization. |
| Gold Nanoparticles (AuNPs) | Nanomaterial that enhances electron transfer, biocompatibility, and catalytic properties [43]. | Incorporated into nanocomposites to improve biosensor sensitivity and performance. |
| 5,5'-Dithio-bis-(2-nitrobenzoic acid) (DTNB) | Known as Ellman's reagent; used in spectrophotometric assays for thiocholine [45]. | Can be used for validating enzyme activity and inhibition independently of electrochemistry. |
The immobilization of acetylcholinesterase (AChE) on electrodes represents a cornerstone in the development of robust electrochemical biosensors for detecting neurotoxic agents such as organophosphorus (OP) pesticides and carbamates [1] [46]. These compounds exert their toxicity through the irreversible inhibition of AChE, a key enzyme in the nervous system, leading to the accumulation of the neurotransmitter acetylcholine and subsequent neurological dysfunction [1] [46]. Conventional analytical methods for pesticide detection, including gas chromatography and high-performance liquid chromatography, are characterized by high accuracy but require sophisticated instrumentation, extensive sample preparation, and trained personnel, limiting their use for rapid, on-site screening [1] [46].
Enzymatic electrochemical biosensors overcome these limitations by offering high sensitivity, rapid response, portability, and cost-effectiveness [1] [23]. The performance of these biosensors is critically dependent on the method used to immobilize the AChE enzyme onto the transducer surface, which directly influences the biosensor's stability, sensitivity, and reproducibility [1] [47]. This application note, framed within a broader thesis on AChE immobilization techniques, details the operational principles, key performance metrics of recent sensor configurations, and standardized protocols for fabricating and operating these analytical devices.
Principles of Detection
Biochemical Basis
AChE (EC 3.1.1.7) catalyzes the hydrolysis of the neurotransmitter acetylcholine into acetate and choline, a process crucial for terminating synaptic transmission [1] [46]. Organophosphorus and carbamate pesticides act as irreversible or reversible inhibitors of AChE, respectively. They form a stable covalent bond with the serine residue within the enzyme's active site, preventing it from functioning [1] [46]. The core principle of AChE-based biosensors is to transduce the degree of this enzymatic inhibition into a quantifiable electrochemical signal that is inversely proportional to the concentration of the pesticide inhibitor [1].
Common Signaling Strategies
Most AChE biosensors utilize acetylthiocholine (ATCh) as a substrate analog. In an uninhibited system, AChE hydrolyzes ATCh to produce thiocholine (TCh) and acetic acid. Thiocholine is an electroactive species that can be oxidized at the electrode surface, generating a measurable anodic current [1] [29].
The core signaling reaction is: Acetylthiocholine + H₂O → Thiocholine + Acetic Acid [28]
The presence of an OP or carbamate pesticide inhibits AChE, leading to a reduction in TCh production and a corresponding decrease in the oxidation current [1]. This "signal-off" approach is the most direct method.
More recent strategies have been developed to improve sensitivity and reduce interference. One innovative "signal-on" approach exploits a charge repulsion effect [13]. In this design, a positively charged electrochemical probe (e.g., Ru[(NH₃)₆]³⁺) is used. The production of positively charged TCh repels the probe from the electrode interface, resulting in a low signal. When the enzyme is inhibited by a pesticide, less TCh is produced, the repulsion effect is weakened, and the electrochemical signal is restored, providing a "signal-on" response that can offer a lower background [13].
The following diagram illustrates the primary signaling pathways and the charge repulsion mechanism used in AChE biosensors for pesticide detection.
Performance Comparison of Recent AChE Biosensors
The selection of immobilization support and transducer design profoundly impacts the analytical performance of AChE biosensors. The table below summarizes key figures of merit for recently reported configurations, highlighting the diversity of materials and their effectiveness.
Table 1: Performance Metrics of Selected AChE-Based Biosensors for Pesticide Detection
| Immobilization Platform/ Electrode | Target Analyte | Detection Principle | Linear Range | Detection Limit | Reference |
|---|---|---|---|---|---|
| MnMOF on FTO electrode [13] | Chlorazophos (OP) | Charge repulsion using Ru[(NH₃)₆]³⁺ | Not specified | 0.532 ng/mL | [13] |
| Functionalized Renewable Carbon (RCF) on GCE [23] | Carbaryl (Carbamate) | Amperometric (TCh oxidation) | 5.0 - 30.0 nmol L⁻¹ | 4.5 nmol L⁻¹ | [23] |
| Flow-through cell with P[5]A/MB/Thionine modified SPCE [29] | Carbofuran (Carbamate) | Amperometric mediation via pillar[5]arene | 10 nM - 0.1 μM | 10 nM | [29] |
| Prussian Blue/Cu-NPs modified electrode [20] | Broad-spectrum insecticides | Amperometric | Not specified | Not specified | [20] |
Detailed Experimental Protocols
Protocol 1: Fabrication of an AChE Biosensor Based on a Metal-Organic Framework (MnMOF) Platform
This protocol describes the construction of a highly stable biosensor where AChE is immobilized within a squaric acid-based MnMOF, utilizing a charge repulsion mechanism for sensitive detection of OPs [13].
Research Reagent Solutions
Table 2: Essential Reagents for AChE@MnMOF Biosensor Fabrication
| Reagent/Material | Function/Description |
|---|---|
| Acetylcholinesterase (AChE) | Biorecognition element; catalyzes the hydrolysis of ATCh. |
| Squaric Acid (and Sodium Salt) | Organic ligand for constructing the Metal-Organic Framework (MOF). |
| Manganese Chloride Tetrahydrate (MnCl₂·4H₂O) | Metal ion source for MOF coordination. |
| Acetylthiocholine Chloride (ATCl) | Enzyme substrate; hydrolysis product (TCh) is key to signal generation. |
| Ru[(NH₃)₆]³⁺ | Positively charged electrochemical probe; signal is modulated by TCh repulsion. |
| Fluorine-doped Tin Oxide (FTO) Electrode | Transducer substrate for immobilizing the AChE@MnMOF composite. |
| Phosphate Buffered Saline (PBS), pH 7.0 | Reaction medium for enzymatic hydrolysis and electrochemical measurement. |
Step-by-Step Procedure
Synthesis of AChE@MnMOF:
- Prepare a 0.1 M solution of squaric acid sodium salt in ultrapure water by dissolving squaric acid in a 0.2 M NaOH solution with the aid of ultrasound.
- Add 1 mg of AChE to 0.5 mL of the prepared squaric acid sodium salt solution.
- In a separate vial, prepare a 0.1 M solution of manganese chloride tetrahydrate in a 15% ethanol solution.
- Mix the two solutions and allow the reaction to proceed at room temperature for 24 hours to form the AChE-encapsulated MnMOF (AChE@MnMOF).
- Centrifuge the resulting suspension, discard the supernatant, and wash the precipitate with deionized water to remove unreacted components. The obtained AChE@MnMOF is ready for electrode modification [13].
Electrode Modification:
- Clean the FTO electrode sequentially with acetone, ethanol, and deionized water in an ultrasonic bath, then dry under a stream of nitrogen gas.
- Prepare a homogeneous suspension of the AChE@MnMOF in water.
- Drop-cast a precise volume (e.g., 5-10 µL) of the AChE@MnMOF suspension onto the active surface of the FTO electrode.
- Allow the electrode to dry thoroughly at room temperature to form a stable film [13].
Electrochemical Detection of OPs:
- Assemble a standard three-electrode system with the modified FTO as the working electrode, an Ag/AgCl reference electrode, and a platinum wire counter electrode. Use a solution containing 0.1 M KCl and the Ru[(NH₃)₆]³⁺ probe in a phosphate buffer.
- Incubation and Inhibition: Immerse the modified electrode in a sample solution containing the target OP pesticide (e.g., chlorazophos) for a fixed period (e.g., 10-15 minutes). The OP will inhibit the AChE within the MOF.
- Signal Measurement: Transfer the electrode to the electrochemical cell containing the probe and the substrate ATCl. The enzymatic reaction is allowed to proceed for a set time. The cathodic current of the Ru[(NH₃)₆]³⁺ probe is measured, typically at a potential around -0.25 V vs. Ag/AgCl. The signal recovery is proportional to the OP concentration due to reduced TCh production and weakened charge repulsion [13].
The following workflow visualizes the key steps of biosensor fabrication and the detection process.
Protocol 2: Fabrication of a Flow-Through AChE Biosensor with Electropolymerized Mediators
This protocol outlines the assembly of a disposable, flow-through biosensor ideal for automated or sequential analysis, using a 3D-printed cell and a screen-printed electrode modified with carbon black and electropolymerized mediators [29].
Key Steps
Modification of the Screen-Printed Carbon Electrode (SPCE):
- Prepare a dispersion of carbon black (CB) in dimethylformamide (DMF).
- Drop-cast the CB suspension onto the working electrode of the SPCE and dry.
- Incubate the CB-modified SPCE with a solution of pillar[5]arene (P[5]A) to adsorb the macrocycle onto the high-surface-area carbon material.
- Electropolymerize a mixture of Methylene Blue (MB) and Thionine onto the modified electrode by performing multiple cyclic voltammetry (CV) scans in a phosphate buffer solution containing the dyes. This creates a stable, mediating polymeric film [29].
Immobilization of AChE in the Flow Cell:
- Fabricate a flow cell reactor (e.g., by 3D printing using poly(lactic acid)).
- Activate the inner walls of the reactor cell with a mixture of N-hydroxysuccinimide (NHS) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) to create reactive esters for covalent binding.
- Flush the activated reactor with a solution of AChE, allowing the enzyme to covalently immobilize on the inner surface. Wash with buffer to remove any unbound enzyme [29].
Flow-Injection Analysis of Inhibitors:
- Integrate the AChE-reactor and the modified SPCE into the flow-through system.
- Pump a carrier buffer stream through the system.
- Inject a defined volume of the substrate (ATCh) solution. The ATCh is hydrolyzed to TCh as it passes through the enzyme reactor.
- The TCh product then flows to the electrode, where it is oxidized via electrocatalysis by the P[5]A/MB/Thionine layer, generating a steady-state baseline current.
- For inhibitor determination, the sample containing the pesticide is injected and allowed to incubate in the system (by stopping the flow or using a longer loop), inhibiting the immobilized AChE.
- A subsequent injection of ATCh yields a decreased current response, the magnitude of which is proportional to the inhibitor concentration [29].
The immobilization of AChE on electrodes is a mature yet rapidly evolving technology that provides a reliable and practical solution for the sensitive detection of neurotoxic pesticides. The integration of advanced nanomaterials, such as MOFs and functionalized carbon platforms, along with innovative detection strategies like charge repulsion, continues to push the boundaries of sensitivity, stability, and operational convenience [1] [13] [23]. The protocols detailed herein offer researchers reproducible methodologies for fabricating high-performance biosensors. These tools are invaluable for environmental monitoring, food safety assurance, and toxicological research, enabling the rapid screening of hazardous substances outside conventional laboratory settings.
Within drug discovery, particularly for neurodegenerative diseases like Alzheimer's, acetylcholinesterase (AChE) is a critical therapeutic target. The development of AChE inhibitors from natural sources represents a promising avenue for identifying new lead compounds. AChE immobilization on electrodes is a key enabling technology, forming the basis of biosensors that allow for rapid, sensitive, and high-throughput screening of potential inhibitors. This application note details how immobilized AChE systems are engineered and deployed to efficiently screen natural product libraries, bridging advanced sensor technology with the discovery of next-generation therapeutics.
Key Applications of Immobilized AChE in Inhibitor Screening
Immobilized AChE biosensors are primarily used in two key screening paradigms: inhibition-based assays for functional activity screening and affinity-based studies for binding interaction analysis. The table below summarizes the core applications, their underlying principles, and the quantitative data they yield, which are crucial for lead compound identification.
Table 1: Key Screening Applications of Immobilized AChE Biosensors
| Application | Principle | Measured Output | Key Quantitative Data |
|---|---|---|---|
| Functional Inhibition Assay [38] [18] | Measures the decrease in AChE catalytic activity upon inhibitor binding. | Reduction in electrochemical or optical signal generated by the enzymatic reaction. | - Inhibition Constant (IC₅₀)- Percentage Inhibition |
| Affinity & Binding Characterization [48] | Probes the stability and nature of the enzyme-inhibitor complex. | Changes in binding site conformation or complex stability monitored via biosensor response. | - Binding Affinity- Complex Stability |
Experimental Protocols for Inhibitor Screening
This section provides detailed methodologies for constructing an AChE-immobilized biosensor and performing inhibition assays, which are fundamental for natural product screening.
Protocol: Construction of an AChE@MnMOF-Modified Electrode
This protocol describes the synthesis of a metal-organic framework (MOF) enzyme immobilization platform and its application in electrode modification for enhanced stability [13].
Synthesis of AChE@MnMOF:
- Prepare a 0.1 M solution of squaric acid sodium salt by dissolving squaric acid in a 0.2 M NaOH solution with ultrasound.
- Add 1 mg of AChE to 0.5 mL of the prepared squaric acid sodium solution.
- Prepare a 0.1 M solution of manganese chloride tetrahydrate in a 15% ethanol solution.
- Mix the two prepared solutions and let the mixture react at room temperature for 12 hours.
- Recover the resulting AChE@MnMOF composite via centrifugation, wash it three times with deionized water, and re-disperse it in 1 mL of water.
Electrode Modification:
- Clean and pre-treat a Fluorine-doped Tin Oxide (FTO) electrode.
- Deposit 8 µL of the AChE@MnMOF suspension onto the FTO electrode surface.
- Allow the electrode to dry at room temperature to form a stable film.
Protocol: Inhibition Assay for Natural Product Screening
This protocol outlines the steps for using the constructed biosensor to evaluate the inhibitory potential of natural compounds or plant extracts [38].
Baseline Activity Measurement:
- Immerse the AChE-modified biosensor in an electrochemical cell containing a suitable buffer (e.g., phosphate buffer, pH 7.4) and the electrochemical probe (e.g., Ru(NH₃)₆³⁺).
- Add the enzyme substrate, acetylthiocholine iodide (ATCh), to a final concentration of 0.36 mM.
- Record the amperometric or voltammetric signal to establish the baseline current response (I₀).
Inhibitor Incubation:
- Incubate the biosensor with the natural product extract or purified compound solution for a fixed period (e.g., 10-15 minutes). This allows potential inhibitors to bind to the immobilized enzyme.
Inhibited Activity Measurement:
- Rinse the biosensor gently with buffer to remove unbound compounds.
- Re-immerse the biosensor in a fresh solution containing buffer and substrate identical to step 1.
- Record the new electrochemical signal (Iᵢ).
Data Analysis:
- Calculate the percentage of enzyme inhibition using the formula: Inhibition (%) = [(I₀ - Iᵢ) / I₀] × 100
- A dose-response curve can be generated by testing a range of inhibitor concentrations to determine the IC₅₀ value.
The Scientist's Toolkit: Research Reagent Solutions
The table below lists key reagents and materials essential for developing AChE-based biosensors for inhibitor screening.
Table 2: Essential Research Reagents for AChE Inhibitor Screening Biosensors
| Reagent/Material | Function/Description | Application in Screening |
|---|---|---|
| Acetylcholinesterase (AChE) | The target enzyme, often immobilized to enhance stability and reusability. | Primary biological recognition element. |
| Metal-Organic Frameworks (MOFs) | Nanomaterial carriers for enzyme immobilization [13]. | Enhance enzyme loading, stability, and signal response. |
| Screen-Printed Carbon Electrodes | Disposable, planar electrochemical cells [38] [28]. | Provide a robust, low-cost, and portable platform for biosensor fabrication. |
| Acetylthiocholine Iodide | Synthetic substrate for AChE [28]. | Hydrolyzed to produce electroactive thiocholine, generating the measurable signal. |
| Electrochemical Probe | Mediator that facilitates electron transfer [13]. | Enables sensitive detection of enzymatic activity; charge repulsion with TCh forms the basis of "turn-on" sensors. |
| Natural Product Libraries | Collections of purified compounds or crude extracts from plants, fungi, or marine organisms [49] [50]. | Source of potential AChE inhibitors for screening. |
Workflow and Signaling Pathways
The following diagrams illustrate the core signaling principle of a charge-repulsion AChE biosensor and the integrated experimental workflow for screening natural product inhibitors.
AChE Biosensor Signaling Principle
This diagram visualizes the "turn-on" signaling mechanism based on charge repulsion, used to detect AChE activity and its inhibition [13].
Natural Product Inhibitor Screening Workflow
This flowchart outlines the end-to-end experimental process, from biosensor preparation to data analysis for inhibitor discovery.
Overcoming Immobilization Challenges: Strategies for Enhanced Performance and Stability
Enzyme leaching—the unwanted release of immobilized enzymes from their solid supports—poses a significant challenge in developing robust biosensors and biocatalytic systems. This technical review examines advanced immobilization techniques for acetylcholinesterase (AChE), a crucial enzyme for neurotransmitter regulation and pesticide detection. Within the broader context of electrode-based biosensor research, selecting an appropriate immobilization strategy is paramount for achieving optimal analytical performance, operational stability, and resistance to enzyme leakage. The following sections provide a comparative analysis of contemporary methodologies, detailed experimental protocols, and performance metrics to guide researchers in selecting immobilization approaches that effectively mitigate enzyme leaching while maintaining enzymatic activity.
Comparison of Immobilization Techniques
The selection of an immobilization technique involves balancing multiple factors including binding strength, enzyme activity retention, and operational stability. The following table summarizes the key characteristics of four prominent AChE immobilization methods, with a specific focus on their capacity to prevent enzyme leaching.
Table 1: Performance Comparison of Acetylcholinesterase Immobilization Techniques
| Immobilization Technique | Support Material | Binding Mechanism | Key Performance Advantages | Impact on Enzyme Kinetics |
|---|---|---|---|---|
| Physical Adsorption [45] | Mesoporous Silicon | Physical forces (van der Waals, hydrophobic) | Enhanced pH (4-9) and thermal stability (up to 90°C); Reusable for 3 cycles; 44-day shelf life [45]. | Not specified |
| Covalent Bonding [28] | Screen-Printed Carbon Electrodes (SPCE) | Covalent bonds between enzyme and functionalized surface | High reproducibility (RSD < 4.0%); Good operational stability [28]. | Not specified |
| Entrapment in Biopolymers [51] | Alginate/κ-Carrageenan blend | Physical entrapment in a polymer network | Improved storage and thermal stability; Wider pH activity profile; Easy and low-cost procedure [51]. | ( Km ): 39.7-52.9 mM min⁻¹ (Immobilized) vs. 50.0 mM min⁻¹ (Free); ( V{max} ): 8.68-12.7 mM (Immobilized) vs. 6.35 mM (Free) [51]. |
| Metal-Organic Framework (MOF) [13] | MnMOF (Squaric acid-based) | Coordination and physical confinement | Superior storage stability & resistance to harsh conditions (temperature, organic solvents); Lower detection limit for pesticides [13]. | Not specified |
Table 2: Quantitative Stability Metrics of Immobilized Acetylcholinesterase
| Technique | Retained Activity After Immobilization | Thermal Stability | Reusability (Cycles) | Shelf-Life (Days) |
|---|---|---|---|---|
| Physical Adsorption [45] | High catalytic behavior | Stable up to 90°C | 3 | 44 |
| Entrapment [51] | High activity retention | Increased | Not specified | Not specified |
| MOF-Based [13] | High activity retention | Superior resistance | Not specified | Not specified |
Experimental Protocols
Protocol 1: Physical Adsorption on Mesoporous Silicon
This protocol describes the immobilization of AChE onto a mesoporous silicon surface via physical adsorption, a method noted for its simplicity and ability to enhance enzyme stability [45].
Materials and Instrumentation
- Porous Silicon Chips: p-type silicon wafers (1x1 cm²), electrochemically anodized in HF-based electrolyte [45].
- Enzyme Solution: Acetylcholinesterase (0.03 units/mL) from human erythrocytes [45].
- Key Reagents: Acetylthiocholine iodide (substrate), 5,5′-dithio-bis(2-nitrobenzoic acid) (DTNB, chromogen), neostigmine methyl sulfate (inhibitor) [45].
- Characterization Equipment: Field Emission Scanning Electron Microscope (FE-SEM), Fourier Transform IR spectroscopy (FT-IR) [45].
Step-by-Step Procedure
- Silicon Wafer Preparation: Cut a boron-doped p-type silicon wafer into 1x1 cm² chips. Degrease by sonication in acetone for 5 minutes, rinse with deionized water, and dry under a nitrogen stream [45].
- Porous Layer Fabrication: Anodize the silicon wafer at a current density of 20 mA/cm² in a HF:H₂O:C₂H₂OH (1:1:2 v/v) electrolyte for 30 minutes in dark conditions. Remove the sample, rinse with deionized water, and dry with nitrogen [45].
- Enzyme Immobilization: Pipette 20 µL of the AChE solution (0.03 units/mL) onto the surface of the porous silicon and allow it to dry at room temperature, completing the physical adsorption process [45].
- Activity Assay (Ellman's Method):
- Prepare an assay mixture containing 180 µL of 50 mM Tris/HCl buffer (pH 8.0), 0.1 M NaCl, 0.02 M MgCl₂, 20 µL of the immobilized AChE on the chip, and 15 µL of 14.9 µM neostigmine methyl sulfate (if testing inhibition). Pre-incubate for 30 minutes at 4°C [45].
- Add 20 µL of 0.3 mM DTNB and 20 µL of 1.8 mM acetylthiocholine iodide to the mixture. Incubate for 10 minutes at 37°C [45].
- Measure the absorbance at 412 nm using a microplate reader. The hydrolysis product reacts with DTNB to produce 2-nitro-5-thiobenzoate, which can be detected colorimetrically [45].
Protocol 2: Covalent Immobilization on Screen-Printed Electrodes
This protocol outlines the covalent attachment of AChE to screen-printed carbon electrodes (SPCEs), creating a stable biosensor platform with low enzyme leaching [28].
Materials and Instrumentation
- Electrodes: Screen-printed carbon electrodes (SPCEs) [28].
- Activation Reagents: Carboxylic acid-functionalized carbon ink or suitable linkers for covalent bonding [28].
- Equipment: Potentiostat for electrochemical measurements [28].
Step-by-Step Procedure
- Electrode Surface Activation: Activate the carbon working electrode surface of the SPCE to generate reactive groups (e.g., carboxyl groups) for enzyme coupling [28].
- Enzyme Coupling: Immobilize AChE covalently onto the activated SPCE surface. The specific covalent chemistry was not detailed in the source, but common methods involve using EDC/NHS chemistry to form amide bonds between enzyme amines and surface carboxyls [28].
- Biosensor Operation and Arsenic Detection:
- Use the AChE/SPCE biosensor in a solution containing the substrate acetylthiocholine iodide (3.6 × 10⁻⁴ M) in Britton-Robinson buffer (pH 7.0) [28].
- Apply a potential of +0.6 V (vs. Ag/AgCl) to oxidize the enzymatic product, thiocholine [28].
- For inhibitor detection (e.g., As(III)), measure the decrease in the amperometric current ((I)) relative to the current in the absence of inhibitor ((I0)). The difference ((ΔI = I0 - I)) is proportional to the inhibitor concentration [28].
Protocol 3: Entrapment in Alginate/κ-Carrageenan Gel
This protocol employs a biopolymer blend of alginate and κ-carrageenan to entrap AChE, a gentle method that preserves enzyme function and improves stability [51].
Materials and Instrumentation
- Biopolymers: Sodium alginate and κ-carrageenan [51].
- Cross-linking Agent: Calcium chloride solution [51].
- Enzyme: Acetylcholinesterase from Electrophorus electricus [51].
Step-by-Step Procedure
- Polymer Solution Preparation: Prepare solutions of sodium alginate and κ-carrageenan in distilled water [51].
- Enzyme-Polymer Mixture: Mix the free AChE solution with the alginate and κ-carrageenan solutions thoroughly [51].
- Bead Formation and Gelation: Add the enzyme-polymer mixture dropwise into a 0.1 M CaCl₂ solution. The calcium ions cross-link the alginate, forming stable gel beads with the enzyme entrapped within the polymer matrix [51].
- Activity Measurement: Use the entrapped enzyme beads in a standard activity assay, such as Ellman's method, and compare the kinetic parameters ((Km), (V{max})) to those of the free enzyme [51].
Protocol 4: Incorporation into Metal-Organic Frameworks (MOFs)
This advanced protocol describes the synthesis of a Mn-based MOF as a host for AChE under mild conditions, offering exceptional stability against leaching and harsh environments [13].
Materials and Instrumentation
- Ligand: Squaric acid (and sodium salt) [13].
- Metal Salt: Manganese chloride tetrahydrate (MnCl₂·4H₂O) [13].
- Support Electrode: Fluorine-doped Tin Oxide (FTO) electrode [13].
- Electrochemical Probe: Ru[(NH₃)₆]³⁺ [13].
Step-by-Step Procedure
- Preparation of Ligand Solution: Dissolve squaric acid in a 0.2 M NaOH solution to create a 0.1 M sodium squarate solution [13].
- Synthesis of AChE@MnMOF: Add 1 mg of AChE to 0.5 mL of the sodium squarate solution. Mix this solution with 0.5 mL of 0.1 M manganese chloride tetrahydrate (prepared in 15% ethanol). Allow the mixture to react at room temperature to form the enzyme-embedded MOF platform, AChE@MnMOF [13].
- Electrode Modification and Sensor Assembly: Coat the synthesized AChE@MnMOF composite onto a clean FTO electrode [13].
- Detection via Charge Repulsion:
- Immerse the modified electrode in a solution containing the substrate acetylthiocholine chloride (ATCl) and the positively charged electrochemical probe, Ru[(NH₃)₆]³⁺ [13].
- The enzymatic hydrolysis of ATCl produces positively charged thiocholine (TCh), which repels the positively charged Ru[(NH₃)₆]³⁺ probe, leading to a reduced electrochemical signal [13].
- In the presence of an organophosphorus pesticide (OP), AChE activity is inhibited, less TCh is produced, the charge repulsion is weakened, and the electrochemical signal is restored. The signal increase is proportional to the OP concentration [13].
The Scientist's Toolkit: Essential Research Reagents
Successful implementation of the aforementioned protocols requires specific materials and reagents. The following table lists key solutions and their functions in AChE immobilization and biosensing.
Table 3: Key Research Reagent Solutions for AChE Immobilization and Biosensing
| Reagent / Material | Function / Role in Research | Example Application |
|---|---|---|
| Acetylthiocholine Iodide | Synthetic substrate for AChE; hydrolysis product is electroactive. | Activity assay in electrochemical and spectrophotometric biosensors [45] [28]. |
| 5,5′-Dithio-bis(2-nitrobenzoic acid) (DTNB) | Chromogenic agent (Ellman's reagent); reacts with thiocholine to produce a yellow anion. | Spectrophotometric activity measurement of free and immobilized AChE [45] [51]. |
| p-type Silicon Wafers | Substrate for creating high-surface-area mesoporous silicon supports via electrochemical etching. | Platform for physical adsorption of AChE [45]. |
| Sodium Alginate & κ-Carrageenan | Natural biopolymers used to form hydrogels for gentle enzyme entrapment. | Matrix for AChE entrapment, enhancing stability [51]. |
| Squaric Acid & Metal Ions (e.g., Mn²⁺) | Organic ligand and metal source for constructing Metal-Organic Frameworks (MOFs). | Synthesis of a mild-condition MOF for AChE encapsulation [13]. |
| Ru[(NH₃)₆]³⁺ | Positively charged electrochemical probe. | Signal reporter in biosensors based on charge repulsion with enzymatic product [13]. |
Immobilization Workflow and Signal Transduction Mechanisms
The following diagram visualizes the logical workflow for selecting an immobilization technique based on the core research objective, and illustrates the signal transduction mechanism for a representative electrochemical biosensor.
Decision Workflow for Immobilization Technique Selection
Charge Repulsion Signal Transduction in an AChE-MOF Biosensor
Mitigating Mass Transfer Limitations and Conformational Changes
The performance of electrochemical biosensors utilizing acetylcholinesterase (AChE) is critically dependent on the effectiveness of the enzyme immobilization strategy. Two primary challenges dominate this interface: mitigating mass transfer limitations that restrict analyte access to the active site, and controlling conformational changes that can diminish catalytic activity. When enzymes are immobilized with unfavorable orientations or within restrictive matrices, diffusion barriers slow substrate arrival and product removal, while structural deformations alter the enzyme's active site geometry and reduce its catalytic efficiency. This Application Note details optimized protocols that leverage nanomaterial engineering and surface chemistry to simultaneously address both challenges, enabling the development of high-sensitivity biosensors for pharmaceutical and environmental applications. The strategies outlined herein are framed within a broader research thesis on advancing AChE immobilization techniques to create next-generation bioanalytical devices.
Experimental Protocols
Protocol 1: Immobilization on Positively Charged Polypyrrole Nanocomposites
This protocol describes the synthesis of a doped polypyrrole nanocomposite film that creates a positively charged, hydrophilic surface to orient AChE with its active site favorably positioned for efficient electron transfer [43].
Materials Required:
- Pyrrole monomer (double-distilled)
- Indigo carmine (IC) and sodium dodecyl sulfate (DS) as dopants
- Tetrachloroaurate trihydrate (HAuCl₄·3H₂O) for gold nanoparticle (AuNP) formation
- Acetylcholinesterase (AChE, from Electrophorus electricus)
- Acetylthiocholine chloride (ATCl) as enzyme substrate
- Electrochemical cell with standard three-electrode setup
Procedure:
- Electrode Pretreatment: Clean the bare electrode (e.g., glassy carbon) mechanically and electrochemically using standard protocols to ensure a pristine surface.
- Nanocomposite Electropolymerization:
- Prepare a polymerization solution containing 0.1 M pyrrole monomer, 5.0 mM IC, and DS at a concentration above its critical micelle concentration (CMC).
- Using chronoamperometry, apply a constant potential of +0.70 V vs. Ag/AgCl for 200 s to deposit the PPy-IC-DS polymer film onto the electrode surface.
- Immerse the polymer-modified electrode in a 1.0 mM HAuCl₄ solution and perform 15 cyclic voltammetry (CV) scans between -0.20 V and +0.60 V at 50 mV/s to form AuNPs within the polymer matrix, creating the PPy-IC-DS-AuNP nanocomposite.
- Enzyme Immobilization: Incubate the modified electrode with 20 μL of AChE solution (concentration optimized between 0.2–0.5 U/μL) for 60 minutes at 4°C. The enzyme physically adsorbs onto the positively charged, hydrophilic nanocomposite surface.
- Biosensor Storage: Store the finalized AChE/PPy-IC-DS-AuNP biosensor at -15 °C in a dry environment when not in use to maintain enzymatic activity.
Protocol 2: Covalent Immobilization on Amino-Functionalized Carbon Nanotubes (CNT–NH₂)
This protocol utilizes CNTs functionalized with amino groups to electrostatically guide AChE orientation, promoting a favorable conformation that reduces mass transfer barriers and enhances electron transfer [52].
Materials Required:
- Multi-walled carbon nanotubes (MWCNTs)
- 3-aminopropyltriethoxysilane (3-APTES) for amino-functionalization
- Acetylcholinesterase (AChE, from Electrophorus electricus, pI ≈ 5.5)
- Acetylthiocholine chloride (ATCl)
- N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide (EDC) and N-Hydroxysuccinimide (NHS) for covalent coupling (optional variant)
Procedure:
- CNT Functionalization:
- Purify pristine MWCNTs via refluxing in concentrated HNO₃ for 6 h to introduce surface carboxyl groups.
- Suspend the carboxylated CNTs in toluene containing 5% (v/v) 3-APTES and reflux for 12 h under nitrogen atmosphere.
- Filter the resulting amino-functionalized CNTs (CNT–NH₂), wash thoroughly with toluene and ethanol, and dry at 60°C overnight.
- Electrode Modification: Prepare a 1 mg/mL dispersion of CNT–NH₂ in DMF. Deposit 10 μL of this suspension onto a glassy carbon electrode surface and allow it to dry at room temperature, forming a uniform film.
- Enzyme Immobilization: Apply 10 μL of AChE solution (1.0 U/μL in 10 mM PBS, pH 7.4) onto the CNT–NH₂/GC electrode. Incubate for 12 h at 4°C. The negatively charged AChE (surface charge at pH 7.4) spontaneously adsorbs onto the positively charged CNT–NH₂ surface via strong electrostatic interactions, positioning its active site gorge toward the solution.
- Biosensor Stabilization: Rinse the biosensor gently with pH 7.4 buffer to remove loosely bound enzyme. The AChE/CNT–NH₂/GC biosensor is now ready for use and demonstrates high operational stability.
Protocol 3: Organocatalytic Electrochemical Detection of AChE Activity
This protocol presents an alternative detection method for AChE activity that eliminates the need for multiple enzymes, simplifying the system and reducing potential mass transfer limitations in the detection step itself [40].
Materials Required:
- Nortropine-N-oxyl (NNO) organocatalyst
- Acetylcholinesterase (AChE, from Electrophorus electricus)
- Acetylcholine chloride (substrate)
- Phosphate buffer (100 mM, pH 7.4)
- Electrochemical cell with glassy carbon working electrode, platinum wire counter electrode, and Ag/AgCl reference electrode
Procedure:
- Electrochemical Setup: Place the working, reference, and counter electrodes in a water-jacketed glass cell maintained at 37°C with continuous stirring.
- Solution Preparation: Add 10 mL of 100 mM phosphate buffer (pH 7.4) and NNO at an optimal concentration of 5 mM to the electrochemical cell.
- Amperometric Measurement:
- Apply a detection potential of +0.60 V vs. Ag/AgCl.
- Allow the background current to stabilize.
- Add a known concentration of AChE enzyme (50–2000 U L⁻¹ final concentration) to the cell.
- Monitor the increase in catalytic current in real-time as NNO oxidizes the choline produced by AChE-catalyzed hydrolysis of acetylcholine.
- Activity Calculation: Quantify AChE activity from the steady-state current or the initial rate of current increase, using a calibration curve constructed with standard AChE solutions.
Results and Data Presentation
Quantitative Performance of AChE Biosensors
The table below summarizes the analytical performance of biosensors fabricated using the described protocols, highlighting their effectiveness in pesticide detection and activity monitoring.
Table 1: Analytical Performance of Different AChE-Based Biosensors
| Immobilization Matrix | Target Analyte | Linear Range | Detection Limit | Sensitivity | Reference |
|---|---|---|---|---|---|
| PPy-IC-DS-AuNP Nanocomposite | Carbaryl pesticide | 0.05–0.25 ng mL⁻¹ | 0.033 ng cm² mL⁻¹ | -59.5 × 10³ A cm⁻² mL g⁻¹ | [43] |
| Amino-functionalized CNTs (CNT–NH₂) | Paraoxon pesticide | 0.2–30 nM | 0.08 nM | -- | [52] |
| Graphene/Gold Nanoparticle SPCE | Isocarbophos pesticide | 0.1–2000 μg L⁻¹ | 0.012 μg L⁻¹ | -- | [53] |
| NNO Organocatalytic System | AChE Activity | 50–2000 U L⁻¹ | 14.1 U L⁻¹ | -- | [40] |
Characterization of Immobilization Efficiency
Key physicochemical parameters provide insight into the success of the immobilization strategy in mitigating mass transfer and conformational issues.
Table 2: Key Physicochemical Parameters of Immobilized AChE
| Parameter | PPy-IC-DS-AuNP System | CNT–NH₂ System | Significance |
|---|---|---|---|
| Apparent Michaelis-Menten Constant (Kₘ) | -- | 67.4 μM (for ATCh) | Lower Kₘ indicates higher substrate affinity and reduced mass transfer limitations. |
| Langmuir Adsorption Constant | 7.39 × 10⁸ (for carbaryl) | -- | High affinity constant suggests efficient binding of the analyte to the immobilized enzyme. |
| Enzyme Orientation | Active site close to electrode interface | Favourable orientation via electrostatic guidance | Promotes fast direct electron transfer (DET). |
| Storage Stability | Improved at -15 °C | -- | Maintains activity over time, indicating minimal conformational degradation. |
The Scientist's Toolkit: Essential Research Reagents
Table 3: Key Reagent Solutions for AChE Immobilization Experiments
| Reagent / Material | Function / Role in Experiment | Example from Protocols |
|---|---|---|
| Doped Polypyrrole (PPy) | Conducting polymer matrix; enhances electron transfer and provides a scaffold for enzyme attachment. | PPy doped with Indigo Carmine (IC) and Dodecyl Sulphate (DS) [43]. |
| Gold Nanoparticles (AuNPs) | Nanomaterial that boosts electrocatalytic activity, increases surface area, and improves electron transfer. | AuNPs electrosynthesized within the PPy matrix [43] [53]. |
| Functionalized Carbon Nanotubes (CNTs) | Nano-scaffold with high conductivity and large surface area; functional groups control enzyme orientation. | CNTs functionalized with amino groups (CNT–NH₂) for oriented AChE immobilization [52]. |
| Acetylthiocholine Chloride (ATCl) | Synthetic enzyme substrate; hydrolysis product (thiocholine) is electrochemically oxidized. | Used as the substrate to measure AChE activity in amperometric biosensors [43] [52] [53]. |
| Nortropine-N-oxyl (NNO) | Organocatalyst; oxidizes choline electrochemically, enabling direct AChE activity measurement without secondary enzymes. | Used in organocatalytic detection of AChE activity via choline oxidation [40]. |
| Nafion Perfluorinated Resin | Ion-exchange polymer; used as a protective membrane to prevent enzyme leaching and reduce fouling. | Used to encapsulate the AChE/GR/AuNPs modified SPCE sensor [53]. |
Workflow and Signaling Visualizations
AChE Biosensor Fabrication and Inhibition Mechanism
Charge-Guided Enzyme Orientation on Nanomaterials
The performance of acetylcholinesterase (AChE)-based electrochemical biosensors is critically dependent on the precise optimization of experimental conditions during enzyme immobilization and biosensor operation. These conditions directly influence enzymatic activity, stability, and the electron transfer rate between the enzyme and electrode surface, ultimately determining biosensor sensitivity, detection limit, and operational longevity [43]. This application note provides a comprehensive guide to optimizing pH, temperature, and incubation time parameters for AChE biosensors, framed within broader research on AChE immobilization techniques for applications in environmental monitoring, food safety, and drug development.
Experimental Protocols for Condition Optimization
Protocol for Determining Optimal pH
Principle: Enzyme activity and stability are highly dependent on the pH of the immobilization and reaction environment, which affects the ionization state of amino acid residues in the active site.
Materials:
- Acetylcholinesterase (AChE) from electric eel or other sources
- Screen-printed carbon electrodes (SPCEs) or other suitable electrodes
- Britton-Robinson buffer (pH range 5.0-9.0) or phosphate buffers
- Acetylthiocholine chloride (ATCl) or acetylthiocholine iodide as substrate
- Amperometric or voltammetric detection system
Procedure:
- Immobilize AChE on the electrode surface using your chosen method (e.g., cross-linking with glutaraldehyde, entrapment in polymer matrices, or affinity immobilization).
- Prepare buffer solutions across a pH range from 5.0 to 9.0 in 0.5 pH unit increments.
- Measure the amperometric response of each AChE-biosensor to a fixed concentration of substrate (e.g., 0.3-0.5 mM ATCl) in each buffer solution at a constant applied potential (typically +0.6 V vs. Ag/AgCl).
- Plot the measured current response against pH to identify the optimum pH that yields maximum enzymatic activity.
- For inhibition-based biosensors, also evaluate the pH effect on inhibition efficiency by a standard pesticide.
Note: The optimal pH for AChE activity in polypyrrole nanocomposite-based biosensors has been identified at approximately pH 7.0 [28], though this may vary with immobilization matrix.
Protocol for Determining Optimal Temperature
Principle: Temperature affects enzyme activity, stability, and denaturation rates, with implications for both biosensor performance and storage stability.
Materials:
- AChE-immobilized biosensors
- Temperature-controlled water bath or incubator
- Substrate solution (ATCl) in optimal pH buffer
- Electrochemical workstation
Procedure:
- Condition AChE-biosensors in the optimal pH buffer at different temperatures (e.g., 15°C, 25°C, 30°C, 37°C, 45°C).
- Measure the amperometric response to a fixed substrate concentration at each temperature.
- Plot the current response versus temperature to determine the temperature optimum for maximum activity.
- For storage stability assessment, store biosensors at different temperatures (4°C, -15°C, -20°C) and periodically test their activity over time.
- Compare the stability of immobilized AChE with free enzyme under identical conditions.
Note: Studies show that AChE immobilized on MnMOF platforms exhibits superior stability and resistance to temperature treatment compared to free AChE [13]. Storage at -15°C significantly improves biosensor stability [43].
Protocol for Optimizing Incubation Time
Principle: In inhibition-based biosensors, the incubation time with inhibitor (pesticide) directly affects the degree of enzyme inhibition and thus detection sensitivity.
Materials:
- AChE-biosensors
- Standard pesticide solutions (e.g., carbaryl, chlorpyrifos)
- Timer or automated incubation system
Procedure:
- Incubate AChE-biosensors with a fixed concentration of pesticide standard for varying time intervals (e.g., 1, 5, 10, 15, 20, 30 minutes).
- After each incubation period, wash the biosensor gently with buffer to remove unbound pesticide.
- Measure the remaining enzyme activity by recording the amperometric response to a fixed substrate concentration.
- Calculate percentage inhibition for each time point: % Inhibition = [(I₀ - I)/I₀] × 100, where I₀ is the current before inhibition and I is the current after inhibition.
- Plot % inhibition versus incubation time to determine the optimal balance between sensitivity and analysis time.
Note: For organophosphorus insecticides, incubation times of 20 minutes have enabled detection in the range of 10⁻⁸ to 10⁻⁹ M [54]. The micro-electrometric method shows signal stabilization after 10 minutes of incubation [55].
Table 1: Optimized Experimental Conditions for AChE-Based Biosensors
| Parameter | Optimal Range | Specific Examples | Experimental Context |
|---|---|---|---|
| pH | 7.0 - 8.0 | pH 7.0 (Britton-Robinson buffer) [28]pH 8.0 (PBS buffer for micro-electrometric method) [55] | Polypyrrole nanocomposite films [43]; MnMOF platforms [13]; Screen-printed electrodes [28] |
| Temperature | 25°C (operation)-15°C (storage) | 25°C (operational) [55]-15°C (storage) [43]37°C (compared for activity) [55] | Cholinesterase activity assessment [55]; Biosensor storage stability [43] |
| Incubation Time | 10 - 20 minutes | 10 minutes (micro-electrometric method) [55]20 minutes (OP insecticide detection) [54] | Pesticide detection in inhibition-based biosensors [54] [55] |
| Substrate Concentration | 0.3 - 0.5 mM | 3.6 × 10⁻⁴ M ATCl [28]4.03-16.13 mM (optimization range) [55] | Acetylthiocholine hydrolysis [28]; Micro-electrometric method development [55] |
Table 2: Impact of Optimization on Biosensor Performance Characteristics
| Performance Metric | Impact of pH Optimization | Impact of Temperature Optimization | Impact of Incubation Time Optimization |
|---|---|---|---|
| Sensitivity | Maximum activity at pH 7.0-8.0 [28] [55] | Enhanced activity at 25-37°C [55] | Increased inhibition with longer incubation up to plateau [54] |
| Detection Limit | Improved low-end detection with proper pH control [43] | Stable baseline at controlled temperatures [43] | Lower detection limits with optimized incubation [54] |
| Stability | Maintained activity at physiological pH [13] | Significantly improved storage stability at -15°C [43] | Consistent inhibition measurements [55] |
| Reproducibility | RSD < 4% at optimal pH [28] | Reduced variability with temperature control | Reliable inhibition kinetics with standardized times [54] |
Experimental Workflows and Relationships
Diagram 1: Experimental Optimization Workflow for AChE Biosensors. This workflow illustrates the sequential optimization of key parameters in AChE biosensor development, from enzyme immobilization through performance evaluation to final application.
Diagram 2: Interrelationship Between Optimization Parameters and Biosensor Performance. This diagram illustrates how pH, temperature, and incubation time collectively influence critical biosensor performance metrics including enzyme activity, detection limits, and reproducibility.
The Scientist's Toolkit: Essential Research Reagents and Materials
Table 3: Key Research Reagent Solutions for AChE Biosensor Development
| Reagent/Material | Function/Purpose | Example Applications |
|---|---|---|
| Acetylcholinesterase (AChE) | Biological recognition element; catalyzes acetylcholine hydrolysis | Electric eel AChE used in polypyrrole nanocomposite biosensors [43] |
| Acetylthiocholine (ATCl/ATCh) | Enzyme substrate; produces electroactive thiocholine | Measurement of enzymatic activity in biosensors [28] [55] |
| Screen-Printed Electrodes (SPEs) | Disposable electrode platforms; enable mass production | Covalent immobilization of AChE for pesticide detection [28] |
| Polypyrrole (PPy) Nanocomposites | Conducting polymer matrix; enhances electron transfer | PPy-IC-DS-AuNP films for improved catalytic performance [43] |
| Glutaraldehyde | Cross-linking agent; stabilizes enzyme immobilization | Enzyme cross-linking in chitosan matrices [56] |
| Metal-Organic Frameworks (MOFs) | Nanomaterial carriers; provide high surface area for immobilization | MnMOF platforms for AChE immobilization with enhanced stability [13] |
| Britton-Robinson Buffer | Versatile buffer system; enables pH optimization studies | pH optimization for AChE-biosensors (pH 5.0-9.0) [28] |
| Gold Nanoparticles (AuNP) | Nanomaterial enhancers; improve conductivity and electron transfer | Incorporated in polypyrrole nanocomposites [43] |
| Chitosan | Biopolymer matrix; facilitates enzyme encapsulation | Used with glutaraldehyde for enzyme cross-linking [56] |
| Organophosphorus Pesticides | Analytical targets; inhibit AChE activity | Chlorpyrifos, acephate detection using inhibition biosensors [56] |
The systematic optimization of pH, temperature, and incubation time parameters is fundamental to developing high-performance AChE-based biosensors. The experimental protocols and data summarized in this application note demonstrate that optimal conditions typically fall within pH 7.0-8.0, operational temperatures of 25°C, storage temperatures of -15°C, and incubation times of 10-20 minutes for inhibition-based assays. These optimized parameters collectively enhance biosensor sensitivity, stability, and reproducibility, enabling applications ranging from environmental pesticide monitoring to drug evaluation. The integration of these optimized conditions with advanced immobilization matrices such as polypyrrole nanocomposites and MOFs provides a robust foundation for the next generation of AChE biosensors in both research and commercial applications.
Improving Storage Stability and Operational Half-Life of Biosensors
The performance of acetylcholinesterase (AChE)-based biosensors is critically dependent on the stability of the enzyme immobilization platform. These biosensors are widely employed for detecting organophosphorus pesticides (OPs) and neurodegenerative disease therapeutics, but their practical application is often limited by the inherent instability of free AChE, which is susceptible to environmental factors such as temperature, pH, and organic solvents [13] [1]. Enzyme immobilization on nanomaterial-based platforms has emerged as a powerful strategy to enhance biosensor robustness, extending both storage stability and operational half-life while maintaining high catalytic efficiency and sensitivity [13] [18]. This application note examines recent advances in AChE immobilization techniques, providing structured experimental data and detailed protocols to guide researchers in developing enhanced biosensing platforms.
Comparative Performance of Immobilization Platforms
Recent investigations have demonstrated that nanomaterial-based immobilization significantly outperforms conventional methods. The selection of an appropriate support material and immobilization strategy is paramount for optimizing biosensor performance.
Table 1: Comparative Performance of AChE Immobilization Platforms
| Immobilization Platform | Support Material | Stability Improvement | Detection Limit | Linear Range | Reference |
|---|---|---|---|---|---|
| MnMOF-based platform | Squaric acid-based metal organic framework | >30 days storage stability; Enhanced resistance to temperature/organic solvents | 0.532 ng/mL (chlorazophos) | Not specified | [13] |
| MXene-based composite | Ti3C2Tx-Chitosan/Graphene | 1 month storage at room temperature | 14.45 nM (dichlorvos) | 22.6 nM - 11.31 μM | [57] |
| Silver nanowire-graphene-TiO2 | Hybrid nanocomposite | Excellent reproducibility and storage stability | 7.4 nM (dichlorvos) | 0.036 μM - 22.63 μM | [58] |
| Magnetic nanoparticles | Maghemite with silane modifiers | Reusable platform with good repeatability | 20 nM (carbofuran) | 1.56 - 25 μM | [59] |
| Screen-printed electrodes | Sol-gel, PVA-SbQ, metal-chelate affinity | >6 months storage stability | 10-8 to 10-9 M (paraoxon) | Not specified | [54] |
Metal-organic frameworks (MOFs) represent one of the most promising immobilization platforms due to their high specific surface area, tailor-able pore channels, and excellent stability [13]. The AChE@MnMOF platform demonstrates remarkable preservation of enzymatic activity under harsh conditions, maintaining functionality even after exposure to elevated temperatures and organic solvents that would typically denature free enzyme [13]. Similarly, two-dimensional materials such as MXenes (e.g., Ti3C2Tx) provide large surface areas and exceptional conductivity, further enhancing biosensor sensitivity and stability [57].
The immobilization method itself significantly impacts biosensor performance. Comparative studies of screen-printed electrodes have demonstrated that bioencapsulation in sol-gel composites, immobilization by metal-chelate affinity, and entrapment in photopolymerizable polymers all enable excellent storage stability exceeding six months [54]. Each method preserves enzymatic activity while preventing enzyme leaching, though the optimal approach depends on the specific application requirements and manufacturing constraints.
Experimental Protocols for Enhanced Biosensor Fabrication
AChE Immobilization on MnMOF Platform
Principle: This protocol utilizes a squaric acid-based MnMOF synthesized under mild conditions to preserve AChE activity while providing a stable, high-surface-area support matrix [13].
Reagents:
- Squaric acid (C4H2O4)
- Manganese chloride tetrahydrate (MnCl22O)
- Acetylcholinesterase (AChE) from electric eel (≥1000 units/mg protein)
- Sodium hydroxide (NaOH)
- Ethanol (15% v/v solution)
Procedure:
- Preparation of squaric acid sodium solution: Dissolve 0.1 M squaric acid in 0.2 M NaOH solution using ultrasonic agitation until completely dissolved.
- Enzyme addition: Add 1 mg AChE to 0.5 mL of the prepared squaric acid sodium solution.
- Metal solution preparation: Prepare 0.5 mL of 0.1 M manganese chloride tetrahydrate in 15% ethanol solution.
- Mixing and synthesis: Combine the enzyme-squaric acid mixture with the manganese chloride solution.
- Incubation: Allow the mixture to stand at room temperature for 2 hours to form the AChE@MnMOF composite.
- Electrode modification: Deposit 8 μL of the AChE@MnMOF suspension onto a fluorine-doped tin oxide (FTO) electrode surface and allow to dry at room temperature.
Validation: The successful immobilization can be verified through electrochemical characterization using cyclic voltammetry and comparison of enzymatic activity before and after immobilization [13].
MXene-Chitosan AChE Biosensor Fabrication
Principle: This protocol exploits the high conductivity and surface area of MXene (Ti3C2Tx) combined with the biocompatible film-forming properties of chitosan to create a stable enzyme immobilization matrix [57].
Reagents:
- Ti3C2Tx MXene powder
- Chitosan (medium molecular weight)
- Acetylcholinesterase (AChE)
- Bovine serum albumin (BSA)
- Glacial acetic acid
- N-Methyl-2-pyrrolidinone (NMP)
- Dichlorvos (DDVP) for inhibition studies
Procedure:
- Graphene suspension: Prepare a 0.4 mg/mL graphene suspension in DMF and sonicate for 30 minutes.
- MXene-chitosan composite: Disperse Ti3C2Tx powder in 0.2% chitosan solution to achieve 0.25 mg/mL concentration.
- Electrode preparation: Polish a glassy carbon electrode (GCE) with 0.3 and 0.06 μm alumina powder sequentially, followed by ultrasonic cleaning and nitrogen drying.
- Modification steps:
- Deposit 4 μL of graphene suspension onto GCE surface and allow to dry completely.
- Deposit 4 μL of MXene-chitosan composite onto the graphene-modified surface.
- Finally, deposit 4 μL of AChE solution (5 mg/mL in 1% BSA) and dry overnight at 4°C.
Performance Assessment: The biosensor exhibits a linear detection range for dichlorvos from 22.6 nM to 11.31 μM with a detection limit of 14.45 nM, maintaining stability for one month at room temperature [57].
Signaling Mechanisms and Workflow
AChE-based biosensors for organophosphorus pesticide detection primarily operate on an inhibition mechanism, where the pesticide reduces enzymatic activity, thereby decreasing the production of electroactive thiocholine.
Diagram 1: AChE Biosensor Inhibition Mechanism
The charge repulsion effect provides an advanced signaling mechanism that enhances detection sensitivity. In this approach, positively charged thiocholine (TCh) repels an equally positively charged electrochemical probe (Ru[(NH3)6]3+), thereby reducing the electrochemical signal. Organophosphorus pesticides inhibit AChE activity, reducing TCh production and consequently weakening the charge repulsion effect, which restores the electrical signal [13]. This "turn-on" strategy offers lower background signal and reduced false-positive rates compared to traditional "turn-off" approaches.
Table 2: The Scientist's Toolkit: Essential Research Reagents
| Reagent/Material | Function in Biosensor Development | Application Notes |
|---|---|---|
| Squaric acid-based MOFs | Enzyme immobilization platform with high surface area and stability | Provides exceptional enzyme loading capacity and protection from denaturation [13] |
| MXenes (Ti3C2Tx) | Conductive 2D nanomaterial for signal amplification | Enhances electron transfer kinetics and provides large immobilization surface [57] |
| Chitosan | Biocompatible natural polymer for enzyme encapsulation | Excellent film-forming ability and maintains enzyme activity [57] |
| Prussian blue derivatives | Electron mediators for enhanced electrochemical detection | Enables broad-spectrum insecticide screening in co-immobilized systems [20] |
| Magnetic nanoparticles (Maghemite) | Reusable enzyme support platform | Facilitates easy enzyme recovery and repeated measurements [59] |
| Glutaraldehyde | Cross-linking agent for covalent enzyme immobilization | Stabilizes enzyme attachment but may affect activity [54] |
| Screen-printed electrodes | Disposable sensor substrates for mass production | Enable cost-effective, portable biosensor development [54] |
The strategic immobilization of acetylcholinesterase on advanced nanomaterial platforms represents a cornerstone technology for enhancing biosensor stability and operational longevity. The experimental data and protocols presented herein demonstrate that MOF-based, MXene-composite, and magnetic nanoparticle immobilization systems can significantly extend biosensor shelf-life while maintaining high sensitivity for detecting organophosphorus pesticides and other AChE inhibitors. Future development efforts should focus on standardizing immobilization protocols, exploring novel hybrid nanomaterials, and integrating microfluidic pretreatment systems to further enhance biosensor performance in real-world applications. The continued refinement of these immobilization platforms will accelerate the adoption of AChE-based biosensors in field-deployable analytical devices for environmental monitoring, food safety, and clinical diagnostics.
Strategies for Enhancing Resistance to Environmental Denaturation
Within the broader scope of acetylcholinesterase (AChE) immobilization techniques for electrode-based biosensors, enhancing the enzyme's resistance to environmental denaturation is a critical research frontier. AChE biosensors are widely employed for the detection of pesticides, nerve agents, and various cholinesterase inhibitors, but their performance and practical application are often limited by the inherent instability of the biological recognition element [18] [13]. Free AChE is susceptible to degradation under fluctuations in temperature, pH, and organic solvent exposure, leading to compromised sensor longevity and reliability [13]. Immobilization strategies offer a powerful means to anchor the enzyme to a solid support, not only facilitating its reuse and integration into devices but also profoundly improving its stability against harsh environmental conditions. This protocol outlines established and emerging methodologies to create robust AChE-based biosensors with enhanced operational and storage stability.
Background
The principle of enzyme immobilization for stabilization involves confining the enzyme molecules to a defined space while retaining their catalytic activity. This process can shield the enzyme from denaturing forces, reduce conformational changes, and mitigate aggregation. In the context of electrochemical biosensors, effective immobilization is a dual-purpose strategy: it stabilizes the enzyme and positions it optimally for efficient electron transfer to the electrode surface, which is crucial for sensitive detection [43] [18]. Recent advancements have focused on using nanostructured materials as immobilization matrices. These materials, including metal-organic frameworks (MOFs), conductive polymers, and other nanocomposites, provide high surface areas, biocompatible microenvironments, and enhanced electrical conductivity, collectively contributing to superior enzyme stability and biosensor performance [18] [13] [56].
Comparative Analysis of Immobilization Strategies
The following table summarizes key immobilization strategies and their documented impact on the stability of acetylcholinesterase.
Table 1: Strategies for Enhancing AChE Stability via Immobilization
| Immobilization Strategy | Support Material / Method | Key Stability Enhancements Documented | Reference(s) |
|---|---|---|---|
| Nanomaterial Entrapment | Mn-MOF (Squaric acid-based) | Superior storage stability; retained >50% activity after 44 days; enhanced resistance to temperature and organic solvent treatment. | [13] |
| Nanocomposite Entrapment | Polypyrrole-Indigo Carmine-Dodecyl Sulphate-AuNP | Good intra- and inter-electrode reproducibility; stability improved when stored at lower temperatures (-15 °C). | [43] |
| Covalent Binding | Pre-activated Porous Silica (Perlite) | Significant stability against temperature (17.7-fold at 60°C), urea (2.7-fold), and acetonitrile (1.7-fold); retained 80% activity after 16 batch cycles. | [60] |
| Physical Adsorption | Mesoporous Silicon | Retained 50% activity; promising thermal stability up to 90°C; reusability for 3 cycles; pH stability over 4–9; shelf-life of 44 days. | [45] |
| Polymer Entrapment | Photopolymerisable Polymer (PVA-SbQ) | Storage stability of over 6 months when encapsulated in a polymer film. | [54] |
| Sol-Gel Encapsulation | Silica-based Sol-Gel Composite | Storage stability of over 6 months. | [54] |
Detailed Experimental Protocols
Protocol 1: Immobilization of AChE on an Mn-MOF Platform
This protocol describes the synthesis of a squaric acid-based MnMOF under mild conditions to load AChE, constructing a highly stable enzyme immobilization platform (AChE@MnMOF) [13].
Research Reagent Solutions
- Squaric Acid Sodium Solution (0.1 M): Dissolve squaric acid in a 0.2 M sodium hydroxide (NaOH) solution using ultrasonication.
- Manganese Chloride Solution (0.1 M): Prepare in a 15% (v/v) ethanol solution.
- Acetylcholinesterase (AChE) Solution: Dissolve AChE enzyme in a suitable buffer (e.g., phosphate buffer saline) to the required concentration.
- Phosphate Buffer Saline (PBS, 0.1 M, pH 7.4).
Procedure
- Enzyme-Modified Ligand Preparation: Add 1 mg of AChE to 0.5 mL of the prepared 0.1 M squaric acid sodium solution. Mix gently to combine.
- Mixing and Synthesis: Combine the 0.5 mL of the AChE-squaric acid mixture with 0.5 mL of the 0.1 M manganese chloride tetrahydrate solution.
- Incubation: Allow the mixture to stand at room temperature for 12 hours to facilitate the formation of the AChE@MnMOF composite.
- Washing and Storage: Centrifuge the synthesized AChE@MnMOF, discard the supernatant, and wash the precipitate with deionized water. Re-disperse the final product in 0.5 mL of PBS for storage at 4°C until further use.
Protocol 2: Covalent Immobilization on Pre-Activated Porous Silica
This protocol outlines the covalent attachment of AChE to a porous silica support (perlite) via silanization and glutaraldehyde cross-linking, a method proven to drastically improve thermal and chemical stability [60].
Research Reagent Solutions
- Porous Silica Support (e.g., Perlite).
- Silanizing Agent: (3-Aminopropyl)triethoxysilane (APTES).
- Cross-linking Agent: Glutaraldehyde solution (e.g., 2.5% v/v in buffer).
- Acetylcholinesterase (AChE) Solution.
- Coupling Buffer: 0.1 M phosphate buffer, pH 7.0.
Procedure
- Support Silanization: Activate the porous silica support by incubating it with APTES under appropriate conditions (e.g., in toluene at reflux) to introduce primary amine groups onto its surface.
- Glutaraldehyde Activation: Wash the aminated support and then react it with a 2.5% glutaraldehyde solution for 1 hour at room temperature. This step introduces aldehyde groups for enzyme coupling.
- Enzyme Coupling: Thoroughly wash the activated support to remove excess glutaraldehyde. Incubate it with the AChE solution in coupling buffer for several hours (e.g., 4°C for 12-16 hours) to facilitate covalent Schiff base formation.
- Washing and Storage: Wash the immobilized enzyme preparation extensively with coupling buffer to remove any physically adsorbed enzyme. The final product can be stored in buffer at 4°C.
Workflow and Signaling Mechanisms
The following diagram illustrates the logical workflow for selecting an appropriate immobilization strategy based on the desired stability outcome, integrating the protocols and strategies discussed.
The detection mechanism in AChE-based biosensors often relies on electroactive products. The following diagram details a specific signaling mechanism based on charge repulsion, utilized in the AChE@MnMOF electrochemical sensor.
The Scientist's Toolkit: Essential Research Reagents
Table 2: Key Reagents for AChE Immobilization Protocols
| Reagent / Material | Function / Role in Immobilization | Example from Protocols |
|---|---|---|
| Squaric Acid & Mn²⁺ Ions | Organic ligand and metal ion source for constructing a biocompatible Metal-Organic Framework (MOF) under mild conditions. | AChE@MnMOF Platform [13] |
| Porous Silica (Perlite) | A high-surface-area inorganic support for enzyme attachment, providing mechanical stability. | Covalent Immobilization [60] |
| (3-Aminopropyl)triethoxysilane (APTES) | A silanizing agent that introduces reactive amine (-NH₂) groups onto silica-based surfaces. | Covalent Immobilization [60] |
| Glutaraldehyde | A homobifunctional cross-linker that reacts with amine groups to form stable covalent bonds with enzymes. | Covalent Immobilization [60] |
| Polypyrrole (PPy) & Gold Nanoparticles (AuNPs) | Conductive polymer and nanoparticles used in nanocomposites to enhance electron transfer and provide a matrix for entrapment. | Nanocomposite Entrapment [43] |
| Photopolymerisable Polymer (PVA-SbQ) | A polymer that forms a stable, cross-linked hydrogel matrix upon UV exposure, physically entrapping the enzyme. | Polymer Entrapment [54] |
| Screen-Printed Carbon Electrodes (SPCEs) | Disposable, low-cost electrochemical transducers suitable for mass production and field-deployable biosensor design. | Biosensor Assembly [54] [28] [61] |
Benchmarking Biosensor Performance: Validation, Metrics, and Comparative Analysis
Within the broader scope of our thesis on acetylcholinesterase (AChE) immobilization techniques, the critical importance of standard analytical metrics cannot be overstated. The performance of any developed AChE-based biosensor is quantitatively evaluated through three fundamental parameters: the detection limit (LoD), which defines the lowest detectable analyte concentration; sensitivity, representing the magnitude of signal change per unit concentration change; and the linear range, which specifies the concentration interval over which the sensor response changes linearly with concentration [1]. These metrics collectively determine the practical utility of biosensors for environmental monitoring, food safety, and pharmaceutical applications. This document provides a standardized framework for comparing these critical parameters across different AChE immobilization platforms and details the experimental protocols for their determination.
Performance Comparison of AChE Biosensor Platforms
The immobilization matrix significantly influences the analytical performance of AChE biosensors. Nanomaterials have been extensively employed to enhance electron transfer, increase surface area, and improve enzyme stability. The table below summarizes the reported performance metrics for various AChE-based biosensors documented in recent literature.
Table 1: Analytical performance of different AChE-based biosensors for the detection of inhibitors.
| Immobilization Platform | Target Analyte | Detection Limit | Linear Range | Sensitivity | Citation |
|---|---|---|---|---|---|
| Ti3C2Tx MXene Quantum Dots | Chlorpyrifos (OP) | 1 × 10⁻¹⁷ M | 10⁻¹⁴ – 10⁻⁸ M | Not Specified | [62] |
| NaOH-etched Glassy Carbon Electrode | 3-Nitropropionic Acid | 0.05 μg/L | 0.1 – 30 μg/L | Not Specified | [63] |
| Prussian Blue-Pd-MXene nanocomposite | Berberine | 10.0 nM | 0.13 – 41 μmol/L | Not Specified | [64] |
| Screen-Printed Electrode (GR/AuNPs) | Isocarbophos (OP) | 0.012 μg/L | 0.1 – 2000 μg/L | Not Specified | [53] |
| Carbon Foam/Graphene/Au | Carbofuran | 8.08 μM | 25 – 125 μM | 0.3874 mA μM⁻¹ cm⁻² | [65] |
| Replaceable Enzyme Reactor (Flow-through) | Carbofuran | 10 nM | 10 nM – 0.1 μM | Not Specified | [61] |
| Silver Nanowire–Graphene–TiO₂ | Dichlorvos (OP) | 7.4 nM | 0.036 – 22.63 μM | Not Specified | [58] |
| Cellulose Membrane (Covalent) | Chlorpyrifos (OP) | 5 nM | 15 – 120 nM | Not Specified | [66] |
Detailed Experimental Protocols
Protocol: Fabrication of a Portable SPCE/GR/AuNPs/AChE Biosensor
This protocol outlines the construction of a portable biosensor for field detection of organophosphorus pesticides (OPs), adapted from the work of Wen et al. [53]. The sensor utilizes the synergistic effects of graphene (GR) and gold nanoparticles (AuNPs) for signal enhancement.
3.1.1 Research Reagent Solutions
Table 2: Essential materials and reagents for the SPCE-based biosensor.
| Item | Function / Role |
|---|---|
| Screen-Printed Carbon Electrode (SPCE) | Disposable, portable electrochemical platform. |
| Graphene (GR) dispersion | Nanomaterial for enhancing electrical conductivity and surface area. |
| Gold Nanoparticles (AuNPs) dispersion | Nanomaterial for improving biocompatibility and facilitating electron transfer. |
| Acetylcholinesterase (AChE) enzyme | Biological recognition element that catalyzes substrate hydrolysis. |
| Acetylthiocholine Chloride (ATCl) | Enzyme substrate; hydrolysis product is electrochemically active. |
| Nafion solution | Polymer membrane to encapsulate and stabilize the modified electrode surface. |
| Phosphate Buffered Saline (PBS), pH 7.5 | Electrochemical measurement buffer. |
| Organophosphorus Pesticide Standard | Analyte for inhibition studies (e.g., Isocarbophos). |
3.1.2 Step-by-Step Procedure
- SPCE Pretreatment: Connect the SPCE to a portable potentiostat. Apply 120 μL of PBS (pH 7.5) to the electrode detection area. Perform cyclic voltammetry (CV) scanning from -0.6 V to 1.2 V at a scan rate of 100 mV/s for 20 cycles to activate the electrode and stabilize the surface. Dry the electrode surface with a gentle stream of nitrogen gas [53].
- Nanomaterial Modification: Pipette 10 μL of a well-dispersed GR suspension (optimal concentration determined to be between 0.05–0.35 mg/mL) onto the pretreated working electrode surface. Allow it to dry at room temperature. Subsequently, apply 10 μL of an AuNPs dispersion (optimal concentration 50–200 μg/mL) on top of the GR layer and dry at room temperature [53].
- Enzyme Immobilization: Apply 7.5 μL of AChE solution (activity typically between 0.2–1.4 units) onto the SPCE/GR/AuNPs surface. Dry the electrode in a 4 °C refrigerator to preserve enzyme activity [53].
- Membrane Encapsulation: To prevent leakage of the modifiers and enzyme, apply 5 μL of a 1% Nafion solution to encapsulate the entire modified electrode surface. Dry again in a 4 °C refrigerator. The sensor is now ready for use [53].
- Electrochemical Measurement and Inhibition Assay:
- Record the initial differential pulse voltammetry (DPV) current response (I₀) of the biosensor in PBS (pH 7.5) containing 1 mM ATCl. The DPV parameters are: potential range 0.3–1.1 V, pulse width 0.05 s, pulse period 0.02 s, modulation amplitude 0.05 V [53].
- Incubate the biosensor in a sample solution containing the target OP inhibitor for a specified time (e.g., 10–15 minutes).
- Wash the biosensor gently with PBS and record the DPV current response (I₁) again under the same conditions.
- Calculate the inhibition percentage using the formula: Inhibition (%) = [(I₀ - I₁) / I₀] × 100.
- The inhibition percentage is proportional to the logarithm of the OP concentration, which is used to construct the calibration curve [53].
Protocol: Fabrication of an AChE Biosensor via Covalent Immobilization on Cellulose Membrane
This protocol details a colorimetric biosensor for visual detection of pesticides, based on the covalent immobilization of AChE onto a modified cellulose membrane [66].
3.2.1 Research Reagent Solutions
Table 3: Essential materials and reagents for the cellulose membrane biosensor.
| Item | Function / Role |
|---|---|
| Cellulose Membrane (CM) | High-surface-area, porous support for enzyme immobilization. |
| TEMPO, NaClO, NaBr | Catalytic system for oxidizing hydroxyl groups to carboxyl groups on CM. |
| EDC, NHS | Cross-linking agents for activating carboxyl groups to form amide bonds with enzyme amines. |
| Acetylcholinesterase (AChE) | Biological recognition element. |
| Acetylthiocholine (ATCh) | Enzyme substrate. |
| DTNB (Ellman's reagent) | Chromogenic reagent; reacts with enzymatic product to produce a yellow color. |
| Organophosphorus Pesticide (e.g., Chlorpyrifos) | Analyte for inhibition studies. |
3.2.2 Step-by-Step Procedure
- Carboxylation of Cellulose Membrane:
- Prepare a cellulose membrane (CM) using a sol-gel phase inversion method.
- Oxidize the CM using the TEMPO/NaClO/NaBr system at pH 10–11 for a specific duration. This process converts the primary hydroxyl groups on the cellulose surface to carboxyl groups [66].
- Wash the carboxylated CM thoroughly with deionized water and dry.
- Enzyme Covalent Immobilization:
- Activate the carboxyl groups on the modified CM by immersing it in a solution containing EDC and NHS for 30–60 minutes. This forms an active ester intermediate [66].
- Incubate the activated CM with an AChE solution (e.g., 500 U/mL) for 2 hours at room temperature, allowing the formation of stable amide bonds between the activated carboxyl groups on the CM and the amino groups of the AChE enzyme.
- Rinse the resulting AChE-immobilized cellulose membrane biosensor (CBS) with buffer to remove any physically adsorbed enzyme [66].
- Colorimetric Detection and Inhibition Assay:
- In the absence of inhibitor, incubate the CBS with a solution containing ATCh and DTNB. AChE hydrolyzes ATCh to produce thiocholine (TCh), which reacts with DTNB to generate a yellow-colored product (TNB⁻). The color intensity is proportional to the enzyme activity [66].
- For inhibitor detection, first incubate the CBS with a sample containing the pesticide (e.g., chlorpyrifos) for 10 minutes. Then, add the ATCh and DTNB solution. The pesticide inhibits AChE, reducing the production of TCh and resulting in a less intense yellow color (or no color change) [66].
- The color intensity can be quantified using a scanner or spectrometer, and the degree of inhibition is used for quantitative analysis. The limit of detection for chlorpyrifos using this method has been reported to be as low as 5 nM [66].
Signaling Pathways and Workflow Visualizations
AChE Inhibition Biosensing Mechanism
The following diagram illustrates the core principle of AChE-based biosensors for detecting inhibitors, which involves the enzymatic hydrolysis of a substrate and the subsequent electrochemical or colorimetric detection of the product, a process that is inhibited by the target analytes.
Portable SPCE Biosensor Fabrication Workflow
This workflow outlines the sequential steps involved in fabricating the portable screen-printed carbon electrode (SPCE) biosensor, from pretreatment to final encapsulation.
Assessing Precision and Reproducibility in Complex Matrices
This application note provides a detailed experimental framework for evaluating the precision and reproducibility of acetylcholinesterase (AChE)-based biosensors in complex matrices. The immobilization of AChE on electrode surfaces serves as a critical platform for detecting various analytes, including environmental contaminants and neurotransmitters, through enzyme inhibition assays. We present standardized protocols for biosensor fabrication, analytical characterization, and performance validation in biologically relevant samples. The data demonstrate that optimized immobilization strategies yield biosensors with high reproducibility (RSD < 4%), extended stability (up to 6 months), and excellent precision in complex matrices such as blood serum and environmental water samples, enabling reliable analytical measurements for pharmaceutical and environmental monitoring applications.
Acetylcholinesterase immobilization on electrode surfaces has emerged as a fundamental technology for developing sensitive biosensors across multiple domains, including environmental monitoring, clinical diagnostics, and drug development. These biosensors typically operate on the principle of enzyme inhibition, where target analytes such as pesticides, heavy metals, or neurochemicals modulate AChE activity, resulting in measurable electrochemical signals [67] [43]. The analytical performance of these biosensors—particularly their precision and reproducibility in complex matrices—is heavily influenced by the chosen immobilization strategy and electrode platform. This document provides detailed protocols for fabricating and characterizing AChE-based biosensors, with emphasis on assessing their reliability in challenging sample environments containing potential interferents.
Experimental Protocols
Biosensor Fabrication Methods
Covalent Immobilization on Screen-Printed Carbon Electrodes (SPCEs)
Principle: Covalent bonding provides stable enzyme attachment to electrode surfaces, minimizing leaching and maintaining activity in flowing systems [67].
Reagents and Materials:
- Screen-printed carbon electrodes (SPCEs)
- Acetylcholinesterase (AChE) from electric eel (0.03 units/mL)
- Britton-Robinson buffer (pH 7.0)
- Glutaraldehyde (2.5%) as crosslinking agent
- Acetylthiocholine iodide substrate solution
Procedure:
- Electrode Pretreatment: Clean SPCEs electrochemically by cycling in 0.1 M phosphate buffer (pH 7.0) between -1.0 V and +1.0 V until stable voltammograms are obtained.
- Enzyme Immobilization: Apply 20 μL of AChE solution (0.03 units/mL in Britton-Robinson buffer, pH 7.0) to the working electrode surface.
- Crosslinking: Add 1.8 μL of glutaraldehyde (2.5%) to the enzyme solution and incubate at 4°C for 24 hours to facilitate covalent bonding.
- Storage: Store the fabricated biosensors in Britton-Robinson buffer (pH 7.0) at 4°C when not in use.
Quality Control: Verify successful immobilization through cyclic voltammetry in 1 mM K₃Fe(CN)₆, observing decreased current due to non-conductive enzyme layer formation.
Physical Adsorption on Mesoporous Silicon
Principle: Mesoporous structures with high surface area provide physical confinement for enzymes, preserving native conformation and activity [45].
Reagents and Materials:
- Boron-doped p-type silicon wafers
- Hydrofluoric acid (HF) electrolyte (48% w/w)
- Acetylcholinesterase solution (0.03 units/mL)
- Ethanol and acetone for cleaning
- Nitrogen gas for drying
Procedure:
- Porous Silicon Fabrication: Anodize silicon wafers in HF-based electrolyte (HF:H₂O:C₂H₅OH = 1:1:2 v/v) at 20 mA/cm² for 30 minutes under dark conditions.
- Surface Characterization: Verify pore size (2-50 nm) and film thickness (3-4 μm) using field emission scanning electron microscopy.
- Enzyme Loading: Apply 20 μL of AChE solution (0.03 units/mL) to the porous silicon surface and allow to dry at room temperature.
- Activity Assessment: Confirm immobilized enzyme activity using Ellman's spectrophotometric assay with acetylthiocholine iodide as substrate.
Nanocomposite-based Entrapment on Pencil Graphite Electrodes
Principle: Nanomaterial composites enhance electron transfer efficiency and provide biocompatible environments for enzyme stabilization [68].
Reagents and Materials:
- Pencil graphite electrodes (PGEs)
- Graphene oxide nanosheets (GONS)
- Platinum nanoparticles (PtNPs)
- AChE and choline oxidase (ChO) nanoparticles
- Glutaraldehyde (2.5%) for crosslinking
- Cysteamine dihydrochloride for functionalization
Procedure:
- Electrode Modification: Electrodeposit PtNPs/GONS nanocomposite on PGE surface at constant potential.
- Enzyme Nanoparticle Preparation: Prepare AChE and ChO nanoparticles via desolvation method using ethanol and crosslink with glutaraldehyde.
- Surface Functionalization: Treat enzyme nanoparticles with 0.12 g cysteamine dihydrochloride to introduce amino groups.
- Biorecognition Layer: Immobilize functionalized enzyme nanoparticles on PtNPs/GONS/PGE via physical adsorption.
Precision and Reproducibility Assessment Protocol
Principle: Systematically evaluate analytical performance parameters through repeated measurements across different biosensor batches and operators.
Reagents and Materials:
- Acetylthiocholine iodide substrate (3.6 × 10⁻⁴ M)
- Arsenic(III) standard solutions (1 × 10⁻⁸ to 1 × 10⁻⁷ M)
- Spiked tap water and certified water samples
- Potentiostat with three-electrode configuration
Procedure:
- Repeatability Testing:
- Conduct eight successive amperometric measurements using the same biosensor.
- Condition biosensor at 4°C in Britton-Robinson buffer (pH 7) between experiments.
- Calculate relative standard deviation (RSD) of current responses.
Reproducibility Assessment:
- Fabricate three separate biosensor batches using identical protocols.
- Perform complete calibration for arsenic(III) with each biosensor.
- Determine RSD of calibration slopes across different electrodes.
Complex Matrix Validation:
- Spike tap water samples with known arsenic(III) concentrations (e.g., 1.00 μM).
- Analyze using standard addition method to account for matrix effects.
- Compare measured vs. known concentrations for accuracy determination.
Stability Testing:
- Store fabricated biosensors at 4°C in appropriate buffer.
- Measure residual activity weekly against initial response.
- Calculate percentage activity retention over time.
Data Analysis and Performance Metrics
Quantitative Performance Data
The table below summarizes precision and reproducibility metrics for different AChE immobilization approaches:
Table 1: Precision and Reproducibility Metrics for AChE-Based Biosensors
| Immobilization Method | Repeatability (RSD) | Reproducibility (RSD) | Detection Limit | Linear Range | Stability | Reference |
|---|---|---|---|---|---|---|
| Covalent/SPCE | 3.4% | 4.0% | 1.1 × 10⁻⁸ M As(III) | 1 × 10⁻⁸ to 1 × 10⁻⁷ M | - | [67] |
| Physical Adsorption/Porous Silicon | - | - | - | - | 44 days, 50% activity | [45] |
| Nanocomposite/PGE | - | - | 0.001 μM ACh | 0.001-200 μM | 6 months | [68] |
| NiO NPs-CGR-NF/GCE | - | - | 5 × 10⁻¹⁴ M methyl parathion | 1.0 × 10⁻¹³ to 1 × 10⁻¹⁰ M | - | [69] |
| PPy-IC-DS2-AuNP | 1.8% (intra-electrode) | 3.7% (inter-electrode) | 0.033 ng cm² mL⁻¹ carbaryl | 0.05-0.25 ng mL⁻¹ | Improved stability at -15°C | [43] |
Table 2: Analytical Recovery in Complex Matrices
| Biosensor Type | Sample Matrix | Spiked Concentration | Measured Concentration | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| AChE/SPCE | Tap water | 1.00 μM As(III) | 1.04 ± 0.05 μM | 104 | 4.1 |
| AChENPs-ChONPs/GONS/PtNPs/PGE | Human serum | 5.0 μM ACh | 4.88 μM | 97.6 | 0.7 |
| AChENPs-ChONPs/GONS/PtNPs/PGE | Human serum | 10 μM ACh | 9.65 μM | 96.5 | 0.3 |
Interference Studies
The table below documents the effect of potential interferents on AChE-biosensor performance:
Table 3: Interference Effects on AChE-Biosensor Response
| Interferent | Concentration | Inhibition (%) | Notes |
|---|---|---|---|
| As(III) | 1 × 10⁻⁸ M | Significant | Primary analyte |
| Hg(II) | >2 × 10⁻⁷ M | Significant | Major interference |
| Ni(II) | >2 × 10⁻⁶ M | Moderate | Concentration-dependent |
| Cu(II) | >2 × 10⁻⁶ M | Moderate | Concentration-dependent |
| Other metals (Zn, Cd, Fe, Pb, Cr) | - | Minimal | No significant interference at environmental levels |
The Scientist's Toolkit: Essential Research Reagents
Table 4: Key Research Reagents for AChE Biosensor Development
| Reagent/Material | Function | Specific Application Example |
|---|---|---|
| Acetylcholinesterase (AChE) | Biorecognition element | Electric eel AChE for pesticide detection [43] |
| Screen-printed carbon electrodes (SPCEs) | Disposable platform | Covalent immobilization for field detection [67] |
| Mesoporous silicon | High-surface-area support | Physical adsorption with enhanced stability [45] |
| Graphene oxide nanosheets (GONS) | Nanocomposite matrix | Enhanced electron transfer in neurotransmitter detection [68] |
| Platinum nanoparticles (PtNPs) | Electrocatalytic component | H₂O₂ oxidation/reduction in biosensing [68] |
| Acetylthiocholine iodide | Enzyme substrate | Amperometric signal generation [67] [45] |
| Glutaraldehyde | Crosslinking agent | Covalent enzyme immobilization [67] [68] |
| Nafion polymer | Permselective membrane | Interference rejection in complex matrices [69] |
| Nickel oxide nanoparticles (NiO NPs) | Nanocomposite material | Pesticide detection with enhanced sensitivity [69] |
Experimental Workflows
Biosensor Fabrication and Application
Inhibition-Based Detection Mechanism
The protocols and data presented herein demonstrate that AChE-based biosensors fabricated with optimized immobilization techniques exhibit exceptional precision, reproducibility, and stability in complex matrices. Covalent immobilization on SPCEs achieves RSD values below 4% for both repeatability and reproducibility, while nanocomposite-based approaches provide extended operational stability up to six months. The consistency of analytical recoveries in spiked tap water (97.6-104%) and serum samples (96.5-97.6%) confirms method reliability for real-world applications. These standardized protocols enable researchers to systematically evaluate biosensor performance, facilitating the development of robust analytical platforms for environmental monitoring, clinical diagnostics, and drug development.
Within the context of a broader thesis on biosensor development, the immobilization of acetylcholinesterase (AChE) onto electrodes represents a critical step that directly influences analytical performance, operational stability, and detection capabilities. This practical guide provides a comparative analysis of established and emerging AChE immobilization techniques, focusing on their implementation for electrochemical biosensing applications in drug development and environmental monitoring. The immobilization method profoundly affects key parameters including enzyme loading, activity retention, stability under operational conditions, and ultimately, the sensitivity and reliability of the biosensor for detecting inhibitors such as organophosphorus pesticides, neurotoxic gases, and heavy metals.
Comparative Analysis of Immobilization Techniques
The selection of an appropriate immobilization strategy requires careful consideration of the trade-offs between enzyme activity, stability, and simplicity of fabrication. The following table summarizes the core characteristics of the primary techniques investigated in this thesis research.
Table 1: Comparison of Acetylcholinesterase Immobilization Techniques
| Immobilization Technique | Support Material/Platform | Key Findings & Performance Metrics | Best For |
|---|---|---|---|
| Physical Adsorption [45] [70] | Mesoporous Silicon | Retained 50% activity; Thermal stability up to 90°C; pH stability (4-9); Reusable for 3 cycles; Shelf-life of 44 days [45]. | Reusable biocatalysts; Screening of AChE inhibitors [45]. |
| Covalent Bonding | Screen-Printed Carbon Electrodes (SPCEs) [6] [28] | Detection limit for As(III): 1.1 × 10⁻⁸ M; Good repeatability (RSD < 4%) [6] [28]. | Disposable biosensors for environmental monitoring (e.g., arsenic) [6]. |
| Entrapment in Polymer Matrix [54] | Photopolymerisable Polymer (PVA-SbQ) | Storage stability >6 months; Detection of paraoxon in the 10⁻⁹ M range [54]. | Biosensors requiring long shelf-life and high sensitivity for pesticides [54]. |
| Covalent on Modified PSi [26] | Functionalized Porous Silicon (PSi-COOH, PSi-NH₂) | Enzyme activity is influenced by surface wettability and orientation; Stable covalent attachment [26]. | Fundamental studies on enzyme orientation and surface chemistry [26]. |
| Metal-Organic Framework (MOF) [13] | MnMOF (Squaric acid-based) | Superior storage stability & resistance to harsh environments vs. free AChE; LOD for chlorazophos: 0.532 ng/mL [13]. | Enhancing enzyme stability against temperature/organic solvents; sensitive OPs detection [13]. |
| Biopolymer Entrapment [51] | Alginate/κ-Carrageenan Blend | Improved storage and thermal stability vs. free enzyme; Wider pH activity profile; Easy immobilization procedure [51]. | Low-cost encapsulation; Enzyme reactors [51]. |
| Sol-Gel Composite [71] | Ceramic Packing with MWCNT-Sol-Gel | Highly reproducible system (RSD 1.88-2.13%); Optimization via Response Surface Methodology (RSM) [71]. | Fixed-bed reactor systems; Process optimization [71]. |
Detailed Experimental Protocols
Protocol 1: Physical Adsorption on Mesoporous Silicon
This protocol outlines the procedure for immobilizing AChE via physical adsorption onto a mesoporous silicon (PSi) surface, a method valued for its simplicity and ability to yield a reusable biocatalyst with enhanced stability [45] [70].
Research Reagent Solutions:
- Silicon Wafer: Boron-doped p-type, resistivity 1–20 Ω cm [45].
- HF-based Electrolyte: Hydrofluoric acid (HF), deionized water, and ethanol in a 1:1:2 (v/v/v) ratio [45].
- Anodization Setup: Keithley 2400 source meter and platinum counter electrode [45].
- Enzyme Solution: Acetylcholinesterase from human erythrocytes (0.03 units/μL) in an appropriate buffer [45].
- Activity Assay Reagents: Tris/HCl buffer (pH 8.0), acetylthiocholine iodide (substrate), and DTNB (Ellman's reagent) [45].
Procedure:
- PSi Fabrication: Cut a silicon wafer into 1x1 cm² chips. Clean by sonication in acetone and rinse with deionized water. Dry with N₂ gas [45].
- Electrochemical Etching: Anodize the silicon wafer in the HF-based electrolyte at a constant current density of 20 mA/cm² for 30 minutes in the dark [45].
- PSi Rinsing: Immediately after anodization, remove the sample, rinse thoroughly with deionized water, and dry under a stream of nitrogen gas [45].
- Enzyme Immobilization: Pipette 20 μL of the AChE enzyme solution (0.03 units/μL) directly onto the dry PSi surface and allow it to air-dry, enabling physical adsorption onto the porous matrix [45].
- Activity Verification: Assess the success of immobilization and retained enzyme activity using a spectrophotometric bioassay (e.g., Ellman's method) at 412 nm [45].
Protocol 2: Covalent Immobilization on Screen-Printed Carbon Electrodes
This protocol describes covalent attachment of AChE to Screen-Printed Carbon Electrodes (SPCEs) for developing disposable, highly sensitive biosensors for environmental toxins like arsenic [6] [28].
Research Reagent Solutions:
- Screen-Printed Electrodes: Carbon-based working electrode, silver reference electrode [6] [72].
- Coupling Reagents: Typically, a cross-linker like glutaraldehyde or a carbodiimide (e.g., EDC) with NHS for activating carboxyl groups [54] [26].
- Enzyme Solution: Acetylcholinesterase (e.g., from electric eel) [6].
- Electrochemical Cell Setup: Potentiostat and buffer solutions (e.g., Britton-Robinson buffer, pH 7.0) [6].
Procedure:
- Electrode Pre-treatment: Clean and/or pre-treat the SPCEs as required by the specific covalent chemistry (e.g., electrochemical activation, plasma treatment).
- Surface Activation: Modify the electrode surface to introduce reactive groups. For example, activate carboxylated surfaces with a mixture of EDC and NHS to form an amine-reactive ester [26]. Alternatively, functionalize the surface with a cross-linker like glutaraldehyde.
- Enzyme Coupling: Apply a precise volume of AChE solution onto the activated SPCE surface. Incubate in a humid chamber to allow covalent bonding between the enzyme's amine groups and the activated surface.
- Washing: Rinse the modified electrode thoroughly with a suitable buffer to remove any physically adsorbed, non-covalently bound enzyme.
- Sensor Testing: Characterize the biosensor amperometrically in a standard three-electrode system using acetylthiocholine iodide as the substrate. The oxidation current of the generated thiocholine is measured, typically at an applied potential of +0.6 V (vs. Ag/AgCl) [6].
Protocol 3: Entrapment in a Metal-Organic Framework (MOF)
This advanced protocol involves the one-pot synthesis of a MnMOF around AChE molecules, creating a protective microenvironment that significantly boosts the enzyme's stability for demanding applications [13].
Research Reagent Solutions:
- Ligand Solution: 0.1 M squaric acid sodium salt in ultrapure water [13].
- Metal Solution: 0.1 M manganese chloride tetrahydrate (MnCl₂·4H₂O) in a 15% ethanol solution [13].
- Enzyme: Acetylcholinesterase (1 mg for the scale described) [13].
- Substrate: Acetylthiocholine chloride (ATCl) [13].
Procedure:
- Enzyme-Ligand Mixing: Add 1 mg of AChE to 0.5 mL of the 0.1 M squaric acid sodium solution. Mix gently to avoid denaturation [13].
- MOF Synthesis Initiation: Combine the AChE-ligand mixture with 0.5 mL of the 0.1 M manganese chloride solution to initiate the formation of the AChE@MnMOF composite [13].
- Crystallization: Allow the mixture to stand at room temperature for a defined period to facilitate the growth of the MOF matrix around the enzyme molecules [13].
- Platform Fabrication: Centrifuge the resulting AChE@MnMOF suspension and re-disperse the composite. Then, coat it onto the electrode surface (e.g., FTO) and allow it to dry [13].
- Performance Evaluation: Test the electrochemical performance of the biosensor. The platform is known to exhibit excellent storage stability and resistance to organic solvents, making it suitable for detecting OPs like chlorazophos in complex samples [13].
Experimental Workflow and Decision Pathway
The following diagram illustrates the logical decision-making pathway for selecting an appropriate immobilization technique based on the primary research objective.
Diagram 1: Decision pathway for selecting an acetylcholinesterase immobilization technique.
The Scientist's Toolkit: Essential Research Reagents
Successful execution of the protocols requires specific materials and reagents. The following table lists key solutions and their functions.
Table 2: Essential Research Reagents for AChE Immobilization Studies
| Research Reagent | Function/Application | Example from Protocols |
|---|---|---|
| Acetylcholinesterase (AChE) | The enzyme catalyst, whose immobilized activity is being studied. | From electric eel or human erythrocytes [45] [6] [51]. |
| Porous Silicon (PSi) | A high-surface-area support for physical adsorption and covalent grafting. | Fabricated by electrochemical anodization of a silicon wafer [45] [26]. |
| Screen-Printed Electrodes (SPEs) | Disposable, planar ceramic/plastic supports for mass-produced biosensors. | Carbon working electrode with silver reference electrode [6] [72]. |
| Cross-linking Agents (EDC/NHS) | Activate carboxyl groups to form stable amide bonds with enzyme amines. | Used for covalent attachment on functionalized PSi and SPCEs [54] [26]. |
| Sol-Gel Precursors | Form an inorganic porous matrix for enzyme encapsulation. | Tetraethylorthosilicate (TEOS) or methyltrimethoxysilane (MTMOS) [54] [71]. |
| Metal-Organic Framework Components | Form a protective crystalline matrix around the enzyme. | Squaric acid (ligand) and Manganese ions (metal center) [13]. |
| Acetylthiocholine Iodide/Chloride | Substrate for AChE; hydrolysis produces electroactive thiocholine. | Used in activity assays (Ellman's) and amperometric biosensing [45] [6] [13]. |
| Ellman's Reagent (DTNB) | Chromogenic agent that reacts with thiocholine to produce a yellow anion. | Used for spectrophotometric measurement of AChE activity [45] [51]. |
Validation Against Gold-Standard Methods like Chromatography and Spectrophotometry
Within the broader scope of research on acetylcholinesterase (AChE) immobilization techniques for electrodes, validating novel biosensing approaches against established analytical methods is a critical step in demonstrating analytical credibility. Advanced biosensors leveraging innovative immobilization strategies must undergo rigorous comparison with gold-standard techniques to confirm their accuracy, sensitivity, and reliability for applications in drug development, environmental monitoring, and clinical diagnostics [18]. This document provides structured application notes and experimental protocols for the validation of AChE-based biosensors and assays against chromatographic and spectrophotometric reference methods, specifically designed for researchers and scientists engaged in method development.
Comparative Performance Data of AChE Activity Assays
The selection of an appropriate validation method depends on the specific application, required sensitivity, and available instrumentation. The following tables summarize the key operational and performance characteristics of various established and emerging techniques for assessing AChE activity and inhibition, providing a basis for comparative validation.
Table 1: Performance comparison of quantitative methods for AChE activity and inhibition analysis.
| Method | Detection Principle | Linear Range | Limit of Detection (LOD) | Key Advantage |
|---|---|---|---|---|
| RP-HPLC with UV Detection [73] | Conversion of 1-naphthol acetate to 1-naphthol | Not Specified | Not Specified | Accurate for complex biological samples (e.g., blood); validated per ICH guidelines |
| Electrochemical (NNO-based) [74] | Catalytic oxidation of choline by NNO | 50–2000 U L⁻¹ | 14.1 U L⁻¹ | Real-time monitoring; eliminates need for secondary enzymes |
| Micro-Electrometric [75] | pH change from acetic acid production | Not Specified | Not Specified | Inexpensive; suitable for resource-limited settings; correlates well with Ellman (R²=0.9147) |
| Capillary Electrophoresis with OIMER [76] | Separation and detection of thiocholine | Substrate: 0.05–0.30 mmol/L | Not Specified | High enzyme activity due to oriented immobilization; low sample consumption |
Table 2: Applications and validation outcomes of AChE inhibition assays for pesticide detection and drug discovery.
| Method / Platform | Target Analyte | Validation Outcome / Performance | Application Context |
|---|---|---|---|
| AChE-Cellulose Biosensor [66] | Chlorpyrifos | LOD: 5 nmol/L; Linear Range: 15–120 nmol/L | Selective, visual colorimetric detection in water |
| HPTLC with Image Analysis [77] | Plant extract inhibitors | Validated per ICH guidelines; ImageJ software showed best sensitivity & linearity | High-throughput screening of natural products for AChE inhibitors |
| HPLC-IMER-MS [78] | Natural product inhibitors | Enabled direct qualitative comparison of inhibitory strengths in a mixture | Discovery of new AChE inhibitors (e.g., dihydro-latifaliumin C) from complex plant extracts |
Detailed Experimental Protocols
Protocol 1: Validation of a Novel Biosensor against the Standard Ellman Spectrophotometric Assay
This protocol describes the parallel analysis of AChE-inhibiting samples using a novel biosensor (e.g., an electrochemical or optical platform) and the classic Ellman method to establish correlation.
1. Materials and Reagents
- AChE Enzyme: Commercially sourced (e.g., from Electrophorus electricus).
- Substrate: Acetylthiocholine iodide (ATCh).
- Ellman's Reagent: 5,5′-Dithio-bis-(2-nitrobenzoic acid) (DTNB).
- Buffer: Phosphate Buffered Saline (PBS), 0.1 M, pH 7.4 or 8.0.
- Inhibitors: Standard solutions of known AChE inhibitors (e.g., donepezil, galanthamine) or samples containing potential inhibitors (e.g., pesticide residues).
- Test Biosensor: The novel AChE-based biosensor under validation.
- Spectrophotometer: Equipped with a microplate reader or cuvette holder.
2. Equipment Setup
- For the Ellman assay, use a UV-Vis spectrophotometric microplate reader or a standard spectrophotometer, set to an absorbance wavelength of 412 nm [75].
- For the test biosensor, prepare and calibrate the instrument according to the manufacturer's or developer's specifications.
3. Procedure
- Step 1: Ellman Assay.
- Prepare a reaction mixture containing PBS (pH 8.0), DTNB, and AChE.
- In a microplate or cuvette, add the reaction mixture and a range of concentrations of the inhibitor sample.
- Initiate the reaction by adding the substrate ATCh.
- Monitor the increase in absorbance at 412 nm for 5-10 minutes.
- Calculate the rate of reaction for each inhibitor concentration and determine the percentage inhibition relative to a control (no inhibitor).
- Step 2: Biosensor Assay.
- Following the specific protocol for the test biosensor, measure the sensor's response (e.g., current, voltage, color intensity) to the same range of inhibitor concentrations used in the Ellman assay.
- Ensure the AChE enzyme is immobilized on the biosensor as per the research design.
- Perform measurements in triplicate for each concentration.
- Step 3: Data Correlation.
- Plot the percentage inhibition obtained from the biosensor (y-axis) against the percentage inhibition obtained from the Ellman method (x-axis) for each corresponding inhibitor concentration.
- Perform linear regression analysis. A strong correlation (e.g., R² > 0.90, as demonstrated in [75]) validates the biosensor's performance against the gold standard.
Protocol 2: Cross-Validation Using RP-HPLC for AChE Activity in Biological Samples
This protocol uses RP-HPLC as a reference method to validate a novel biosensor's ability to accurately measure AChE activity in complex matrices like blood.
1. Materials and Reagents
- Biological Sample: Human whole blood or isolated RBCs [73] [75].
- Substrate: 1-Naphthol acetate (1-NA) for HPLC; the respective substrate for the test biosensor (e.g., ATCh).
- Mobile Phase: HPLC-grade water and acetonitrile (e.g., 55:45, v/v, isocratic) [73].
- Standards: 1-Naphthol and 1-naphthol acetate for calibration.
- Equipment: RP-HPLC system with C18 column and UV/PDA detector; test biosensor.
2. Equipment Setup
- HPLC Conditions [73]
- Column: C18 reversed-phase (e.g., 150 x 4.6 mm, 4.5 µm).
- Mobile Phase: Water-Acetonitrile (55:45, v/v, isocratic).
- Flow Rate: 1.0 mL/min.
- Detection Wavelength: 280 nm.
- Injection Volume: 20 µL.
- Column Temperature: 25°C.
3. Procedure
- Step 1: HPLC Analysis.
- Prepare blood samples: dilute whole blood or isolate RBCs via centrifugation.
- Incorate the blood sample (e.g., 10 µL) with the substrate 1-Naphthol acetate in phosphate buffer (pH ~7.0).
- Stop the reaction after a fixed time (e.g., 20 min) by adding acetonitrile.
- Centrifuge to precipitate proteins and inject the supernatant into the HPLC system.
- Quantify the produced 1-naphthol based on a calibration curve. The amount of 1-naphthol produced is directly proportional to AChE activity [73].
- Step 2: Biosensor Analysis.
- Test the same pre-incubated blood sample (or an aliquot) using the novel biosensor according to its specific protocol.
- Step 3: Data Comparison and Validation.
- Express the results from both methods in consistent units (e.g., enzyme activity units per volume).
- Use statistical tests (e.g., paired t-test, Bland-Altman analysis) to assess the agreement between the two methods. The biosensor is considered validated if there is no significant systematic difference from the HPLC reference method.
Workflow and Signaling Pathway Visualization
AChE Biosensor Validation Workflow
The following diagram illustrates the logical workflow for validating a novel AChE biosensor against gold-standard methods, integrating key decision points and techniques described in the protocols.
AChE Catalytic and Inhibition Signaling Pathway
This diagram outlines the core biochemical signaling pathway of AChE catalysis and the mechanism of inhibition by organophosphorus pesticides, which is fundamental to the operation of AChE-based biosensors.
The Scientist's Toolkit: Key Research Reagent Solutions
The following table details essential reagents and materials required for the implementation of AChE activity and inhibition assays, along with their critical functions in the experimental workflows.
Table 3: Essential research reagents and materials for AChE activity and inhibition assays.
| Reagent / Material | Function in Assay | Application Examples |
|---|---|---|
| Acetylcholinesterase (AChE) | Biological recognition element; catalyzes substrate hydrolysis | Immobilized on biosensors [18] [66]; used in free solution for kinetic studies [73] |
| Acetylthiocholine (ATCh) | Ester substrate for AChE; hydrolysis product generates measurable signal | Standard substrate in Ellman's method [66] [75] and electrochemical biosensors [76] |
| DTNB (Ellman's Reagent) | Chromogenic transducer; reacts with thiocholine to produce yellow anion | Spectrophotometric detection of AChE activity at 412 nm [66] [79] [75] |
| Organophosphorus Pesticides | Analytes that inhibit AChE; used for assay calibration and validation | Chlorpyrifos used to validate cellulose membrane biosensor [66] |
| Nortropine-N-oxyl (NNO) | Organocatalyst for electrochemical oxidation of choline | Enables real-time amperometric monitoring of AChE activity without secondary enzymes [74] |
| 1-Naphthol Acetate | Alternative chromogenic substrate for AChE | Used in RP-HPLC method for AChE activity determination in blood [73] |
| Functionalized Materials | Matrices for enzyme immobilization to enhance stability and reusability | Cellulose membranes [66]; Gold Nanoparticles (AuNPs) [76]; Metal-organic frameworks (MOFs) [18] |
Within the broader scope of a thesis on acetylcholinesterase (AChE) immobilization techniques for electrodes, the meticulous characterization of the fabricated biosensing interfaces is paramount. The performance of these biosensors—their sensitivity, stability, and reproducibility—is intrinsically linked to the physical, chemical, and electrochemical properties of the immobilized enzyme layer. This application note details the integrated use of four powerful characterization techniques: Field Emission Scanning Electron Microscopy (FE-SEM), Fourier-Transform Infrared Spectroscopy (FT-IR), X-ray Photoelectron Spectroscopy (XPS), and Electrochemical Impedance Spectroscopy (EIS). These methods collectively provide a comprehensive picture, from the nanostructure and chemical composition of the immobilization matrix to the functional efficacy of the final biosensor, thereby guiding the optimization of robust AChE-based electrodes for applications in drug development and environmental monitoring [45] [26] [80].
Characterization Techniques: Principles and Applications
The following suite of techniques allows researchers to correlate the structure and composition of an AChE-immobilized electrode with its analytical performance.
Field Emission Scanning Electron Microscopy (FE-SEM)
Principle: FE-SEM produces high-resolution images of a sample's surface by scanning it with a focused beam of electrons. The interactions between the electrons and the atoms in the sample generate various signals that reveal information about surface topography and composition.
Application in AChE Immobilization: FE-SEM is indispensable for visualizing the porous structure of matrices used for immobilization, such as mesoporous silicon, and for confirming the successful deposition of enzyme layers. It allows researchers to assess critical parameters like pore size distribution, pore geometry, and uniformity of enzyme coating [45] [26]. For instance, cross-sectional FE-SEM can measure porous film thickness, while top-down views confirm that the pore size is appropriate for entrapping AChE molecules without causing steric hindrance [45] [80].
Fourier-Transform Infrared Spectroscopy (FT-IR)
Principle: FT-IR spectroscopy identifies chemical bonds and functional groups in a molecule by measuring the absorption of infrared light. The resulting spectrum is a molecular "fingerprint" that confirms chemical reactions and surface modifications.
Application in AChE Immobilization: This technique is primarily used to verify the success of each step in the surface functionalization process. For example, after silanization or hydrosilylation of a porous silicon surface, FT-IR can detect the appearance of new peaks corresponding to amine (-NH₂) or carboxyl (-COOH) groups, which are essential for subsequent covalent enzyme attachment [26]. Following AChE immobilization, shifts or changes in the amide I and II bands provide evidence of covalent bonding between the enzyme and the activated surface [26] [66].
X-ray Photoelectron Spectroscopy (XPS)
Principle: XPS is a quantitative technique that measures the elemental composition, empirical formula, and chemical state of the elements within a material. It works by irradiating a sample with X-rays and simultaneously measuring the kinetic energy and number of electrons that escape from the top few nanometers of the surface.
Application in AChE Immobilization: XPS provides definitive evidence of successful AChE immobilization by detecting the presence of nitrogen (N1s signal), an element found in the enzyme's protein structure but not in typical substrate materials like silicon or cellulose [45] [66]. The high surface sensitivity of XPS makes it ideal for analyzing the monolayer-level coverage of enzymes on electrode surfaces.
Electrochemical Impedance Spectroscopy (EIS)
Principle: EIS characterizes the electrical properties of an electrochemical interface by applying a small amplitude alternating voltage over a wide frequency range and measuring the current response. The resulting impedance data is often presented in a Nyquist plot.
Application in AChE Immobilization: EIS is a powerful tool for probing electron transfer kinetics at modified electrode surfaces. After each modification step (e.g., nanomaterial deposition, enzyme immobilization), the change in charge transfer resistance (Rₑₜ) can be monitored using a standard redox probe like [Fe(CN)₆]³⁻/⁴⁻ [81] [82]. A successful AChE immobilization often increases Rₑₜ due to the insulating nature of the protein layer. Furthermore, the inhibition of AChE by organophosphorus pesticides directly affects the Rₑₜ, forming the basis for highly sensitive detection schemes [81] [82].
Experimental Protocols
Workflow for AChE Biosensor Fabrication and Characterization
The following diagram outlines the comprehensive workflow from electrode preparation to final characterization, integrating the key techniques discussed.
Protocol 1: AChE Immobilization on Mesoporous Silicon via Covalent Binding
This protocol is adapted from studies on creating stable AChE biosensors [26] [80].
3.2.1 Materials
- Boron-doped p-type silicon wafers (1–20 Ω cm resistivity)
- Hydrofluoric acid (HF, 48%), Ethanol, Acetone
- Acetylcholinesterase (AChE from human erythrocytes or Electrophorus electricus)
- (3-Aminopropyl)triethoxysilane (APTES)
- Cyanuric chloride or N-hydroxysuccinimide (NHS) / N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide (EDC)
- Toluene (anhydrous)
- Tris-HCl buffer (50 mM, pH 8.0)
3.2.2 Procedure
- Porous Silicon Fabrication: Electrochemically etch the silicon wafer in a HF-based electrolyte (e.g., HF:H₂O:C₂H₅OH = 1:1:2 by volume) at a constant current density (e.g., 20 mA/cm²) for 20-35 minutes. Rinse the resulting porous silicon (PSi) chip with deionized water and dry under a nitrogen stream [45] [80].
- Surface Oxidation: Oxidize the hydrogen-terminated PSi surface in a piranha solution (H₂SO₄:H₂O₂ = 3:1) or by thermal treatment to create a hydroxyl-rich surface.
- Silanization: Incubate the oxidized PSi chip in a 2% (v/v) solution of APTES in anhydrous toluene for 2 hours at room temperature to form an amine-terminated surface (PSi-NH₂). Rinse thoroughly with toluene and ethanol to remove unbound silane [26] [80].
- Surface Activation:
- Path A (Cyanuric Chloride): React the PSi-NH₂ surface with a solution of cyanuric chloride in acetone to create a reactive chlorotriazine group [80].
- Path B (NHS/EDC): Alternatively, if a carboxyl-terminated surface is prepared via hydrosilylation with undecylenic acid, activate it with a mixture of NHS and EDC in buffer for 30 minutes to form NHS-esters [26].
- Enzyme Immobilization: Apply 20 μL of AChE solution (e.g., 0.03 units/mL in Tris-HCl buffer, pH 8.0) onto the activated PSi surface and incubate for 1-2 hours at 4°C in a humid chamber. Rinse gently with cold buffer to remove physically adsorbed enzyme.
- Storage: The immobilized AChE biosensor can be stored dry at 4°C until use.
Protocol 2: Electrochemical Characterization via EIS
This protocol is used to validate the immobilization process and to perform pesticide detection [81] [82].
3.3.1 Materials
- AChE-immobilized working electrode
- PalmSens EmStat Pico or similar potentiostat
- Phosphate Buffered Saline (PBS, 0.1 M, pH 7.4) containing 5 mM K₃[Fe(CN)₆]/K₄[Fe(CN)₆] (1:1 mixture) and 0.1 M KCl.
3.3.2 Procedure
- Setup: Connect the AChE-immobilized electrode as the working electrode in a standard three-electrode cell with a Pt counter electrode and an Ag/AgCl reference electrode.
- Measurement Parameters: In the potentiostat software, configure the EIS experiment with the following parameters:
- DC Bias: 0.22 V (or the open circuit potential)
- AC Amplitude: 5-15 mV
- Frequency Range: 0.1 Hz to 100 kHz (or 20 Hz to 200 kHz) [82]
- Baseline Measurement: Immerse the electrode in the PBS/redox probe solution and run the EIS measurement to obtain a baseline Nyquist plot.
- Inhibition Assay (for pesticide detection):
- Incubate the AChE biosensor in a sample solution containing the suspected inhibitor (e.g., chlorpyrifos) for a fixed time (e.g., 10-15 minutes).
- Rinse the electrode gently with buffer.
- Perform the EIS measurement again under identical conditions.
- Data Analysis: Fit the obtained impedance spectra to an equivalent electrical circuit. The increase in the charge transfer resistance (Rₑₜ) value after inhibition is proportional to the concentration of the pesticide [81] [82].
Data Presentation and Analysis
Table 1: Performance data of AChE biosensors fabricated using different immobilization strategies and characterized with the described techniques.
| Immobilization Matrix | Immobilization Method | Characterization Techniques Used | Key Performance Metrics | Reference |
|---|---|---|---|---|
| Mesoporous Silicon | Physical Adsorption | FE-SEM, FT-IR, XPS, PL | Retained Activity: 50%Thermal Stability: Up to 90°CReusability: 3 cyclesShelf-life: 44 days | [45] |
| Modified Porous Silicon | Covalent (Hydrosilylation) | FT-IR, Contact Angle | Confirmed enzyme activity and controlled orientation via surface wettability. | [26] |
| Modified Porous Silicon | Covalent (Silanization + Cyanuric Chloride) | FE-SEM, EDS, FT-IR, PL | Enhanced PL intensity after immobilization; retained dose-dependent inhibitory response. | [80] |
| Cellulose Membrane | Covalent (EDC/NHS) | SEM, EDS, FT-IR, XPS | LOD for Chlorpyrifos: 5 nMLinear Range: 15-120 nM | [66] |
| GR-Ti₃C₂Tₓ Nanocomposite | Adsorption (Chitosan) | EIS, CV, DPV | LOD for Dichlorvos: 14.45 nMLinear Range: 22.6 nM - 11.31 μMStability: 1 month at room temp | [81] |
Essential Research Reagent Solutions
Table 2: Key reagents and materials for AChE immobilization and biosensor fabrication.
| Reagent/Material | Function/Application | Example Source / Purity |
|---|---|---|
| Acetylcholinesterase (AChE) | Biorecognition element; catalyzes hydrolysis of acetylcholine. | Sigma-Aldrich (from human erythrocytes or Electrophorus electricus) [45] [66] |
| Acetylthiocholine (ATCh) | Enzyme substrate; hydrolysis product is electroactive. | Sigma-Aldrich (≥98.0%) [66] |
| 5,5'-Dithio-bis(2-nitrobenzoic acid) (DTNB) | Colorimetric transducer (Ellman's reagent); reacts with thiocholine. | Sigma-Aldrich (≥98.0%) [45] [66] |
| (3-Aminopropyl)triethoxysilane (APTES) | Silanizing agent; introduces amine groups for covalent immobilization. | Sigma-Aldrich (≥98.0%) [26] [80] |
| NHS / EDC | Carbodiimide crosslinkers; activate carboxyl groups for amide bond formation. | Sigma-Aldrich (≥98.0%) [26] [66] |
| Undecylenic Acid | ω-Alkenoic acid for hydrosilylation; creates carboxyl-terminated surfaces. | Acros Organics (99%) [26] |
| Chitosan | Biopolymer for enzyme encapsulation and electrode modification; provides biocompatibility. | Aladdin Bio-Chem Technology [81] |
The strategic integration of FE-SEM, FT-IR, XPS, and EIS provides an unparalleled, multi-faceted view of acetylcholinesterase immobilization on electrode surfaces. FE-SEM confirms the optimal nanostructure for enzyme hosting, FT-IR and XPS deliver irrefutable chemical evidence of successful surface modification and enzyme attachment, and EIS functionally validates the biosensor's performance and sensitivity. This synergistic analytical approach, as detailed in these application notes and protocols, is fundamental to the rational design and development of highly efficient, stable, and reliable AChE-based biosensors. Such platforms hold significant promise for advancing research in drug development, neuropharmacology, and environmental toxicology.
Conclusion
The strategic immobilization of acetylcholinesterase on electrodes is a cornerstone of modern biosensor technology, directly dictating performance in critical applications from environmental monitoring to clinical diagnostics. Advancements in nanomaterial supports, particularly metal-organic frameworks (MOFs), porous silicon, and gold nanoparticles, have dramatically improved biosensor stability, sensitivity, and practicality. The choice of immobilization method—whether physical adsorption, covalent bonding, or entrapment—presents a clear trade-off between enzyme activity retention and operational robustness. Future progress hinges on developing even more stable and reusable biocatalytic platforms, streamlining fabrication for mass production, and expanding applications into real-time, in-vivo monitoring for personalized medicine. The continued refinement of these immobilization techniques promises to unlock new frontiers in rapid, on-site detection and high-throughput screening, offering profound implications for public health, food safety, and pharmaceutical development.
References
- 1. Acetylcholinesterase Biosensors for Electrochemical ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3872028/]
- 2. Fluorometric and Colorimetric Biosensors for the Assay of ... [https://www.mdpi.com/1424-8220/25/9/2674]
- 3. An Acetylcholinesterase-Based Chronoamperometric ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3821328/]
- 4. Progress of biosensors based on cholinesterase inhibition [https://pubmed.ncbi.nlm.nih.gov/19442145/]
- 5. Twenty years research in cholinesterase biosensors [https://www.sciencedirect.com/science/article/abs/pii/S1389034406000025]
- 6. Immobilization of Acetylcholinesterase on Screen-Printed ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3264471/]
- 7. Immobilization of acetylcholinesterase on screen-printed ... [https://www.sciencedirect.com/science/article/pii/S0003267002005184]
- 8. Nanomaterial-driven electrochemical biosensors for ... [https://www.sciencedirect.com/science/article/abs/pii/S1387700325013371]
- 9. Electrochemical biosensors with right-side-out-oriented cell ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12648092/]
- 10. A Sensitive Electrochemical Cholinesterase-Inhibiting ... [https://www.mdpi.com/2079-6374/15/9/575]
- 11. Immobilized enzymes: a comprehensive review [https://bnrc.springeropen.com/articles/10.1186/s42269-021-00649-0]
- 12. A Comprehensive Guide to Enzyme Immobilization: All You ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11857967/]
- 13. The Construction of Acetylcholinesterase Immobilization on ... [https://www.sciencedirect.com/science/article/abs/pii/S0889157525010567]
- 14. Electrochemical Acetylcholinesterase Sensors for Anti- ... [https://www.mdpi.com/2079-6374/14/2/93]
- 15. Review of research progress in immobilization and chemical ... [https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02791-0]
- 16. Importance of Enzyme Immobilization [https://agscientific.com/blog/importance-of-enzyme-immobilization.html?srsltid=AfmBOoqY4bRSBlLoKPgRowWQqQHpWHijofIO9_1DUXiAzbE6WfPHZhTg]
- 17. Enzyme Immobilization: Its Advantages, Applications and ... [https://www.longdom.org/open-access/enzyme-immobilization-its-advantages-applications-and-challenges-1100443.html]
- 18. Advances in acetylcholinesterase-based biosensing ... [https://www.sciencedirect.com/science/article/abs/pii/S030881462504021X]
- 19. Industrial applications of immobilized enzymes—A review [https://www.sciencedirect.com/science/article/pii/S2468823119304560]
- 20. Acetylcholinesterase and butyrylcholinesterase co ... [https://www.semanticscholar.org/paper/6502df1f1bef9c37670b1ef48f0871fadd6714ab]
- 21. Acetylcholinesterase biosensor based on SnO 2 ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566313003035]
- 22. Enzyme Immobilization on Nanomaterials and Their ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12430663/]
- 23. Acetylcholinesterase Biosensor Based on Functionalized ... [https://www.mdpi.com/2079-6374/12/7/486]
- 24. Acetylcholinesterase immobilization and characterization ... [https://www.sciencedirect.com/org/science/article/pii/S1573493516000151]
- 25. Progress on encapsulation and entrapment of enzymes in ... [https://link.springer.com/article/10.1007/s00449-025-03244-z]
- 26. Comparative investigation of two methods for ... [https://www.sciencedirect.com/science/article/abs/pii/S0169433216329002]
- 27. Covalent Immobilization - an overview [https://www.sciencedirect.com/topics/engineering/covalent-immobilization]
- 28. Immobilization of Acetylcholinesterase on Screen-Printed ... [https://www.mdpi.com/1424-8220/10/3/2119]
- 29. Flow-Through Acetylcholinesterase Sensor with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9496324/]
- 30. Immobilization Techniques in the Fabrication of Nanomaterial ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC3673113/]
- 31. An acetylcholinesterase enzyme electrode stabilized by an ... [https://www.sciencedirect.com/science/article/abs/pii/S1388248106005431]
- 32. Metal-organic frameworks for enzymes immobilization [https://www.sciencedirect.com/science/article/abs/pii/S0010854525007271]
- 33. Metal–organic frameworks as highly effective platforms for ... [https://link.springer.com/article/10.1007/s43153-024-00513-4]
- 34. Enhancing Multi‐Enzyme Cascade Activity in Metal ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12138836/]
- 35. Recent Progress in Enzyme Immobilization to Metal ... [https://www.mdpi.com/1420-3049/30/2/251]
- 36. trade-offs between enzyme activity and MOF stability [https://pubs.rsc.org/en/content/articlelanding/2025/gc/d4gc05154h]
- 37. Article Raising enzymatic stability during biocatalysis with ... [https://www.sciencedirect.com/science/article/pii/S266638642400599X]
- 38. Disposable electrochemical biosensor based on ... [https://pubs.rsc.org/en/content/articlelanding/2025/ay/d4ay02084g]
- 39. Advanced strategies for enzyme–electrode interfacing in ... [https://www.sciencedirect.com/science/article/pii/S0167779924003445]
- 40. Measurement of acetylcholinesterase activity by ... [https://pubs.rsc.org/en/content/articlehtml/2025/ra/d5ra04585a]
- 41. Assessing acetylcholinesterase catalytic activity in the ... [https://www.sciencedirect.com/science/article/pii/S0013468625002932]
- 42. Surface Modification of Screen-Printed Carbon Electrode ... [https://pubmed.ncbi.nlm.nih.gov/38667159/]
- 43. Efficient acetylcholinesterase immobilization for improved ... [https://www.sciencedirect.com/science/article/abs/pii/S0925400521004445]
- 44. Applications of chemically modified screen-printed electrodes ... [https://pubs.rsc.org/en/content/articlehtml/2024/ra/d4ra02470b]
- 45. Acetylcholinesterase immobilization and characterization ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC4793299/]
- 46. Cholinesterases and Engineered Mutants for the Detection ... [https://www.mdpi.com/1424-8220/18/12/4281]
- 47. Acetylecholinesterase-based biosensor electrodes for ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566304003379]
- 48. Acetylcholinesterase as a Multifunctional Target in Amyloid ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12428950/]
- 49. Exploration of natural products for the development ... [https://www.sciencedirect.com/science/article/pii/S240584402500859X]
- 50. Natural acetylcholinesterase inhibitors: A multi-targeted ... [https://www.sciencedirect.com/science/article/pii/S2772417424000268]
- 51. A novel matrix for the immobilization of acetylcholinesterase [https://www.sciencedirect.com/science/article/abs/pii/S0141813005002096]
- 52. Efficient immobilization of acetylcholinesterase onto amino ... [https://www.sciencedirect.com/science/article/abs/pii/S0956566315000068]
- 53. A portable acetylcholinesterase-based electrochemical sensor ... [https://pubs.rsc.org/en/content/articlehtml/2023/ra/d2ra05383g]
- 54. Immobilization of acetylcholinesterase on screen-printed ... [https://www.sciencedirect.com/science/article/abs/pii/S0003267002005184]
- 55. Micro-Electrometric Method for Assessing Cholinesterase ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC11932293/]
- 56. A Sensitive Electrochemical Cholinesterase-Inhibiting ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12467903/]
- 57. Acetylcholinesterase electrochemical biosensors with ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC7190139/]
- 58. An acetylcholinesterase with high biosensor and sensitivity... stability [https://pubs.rsc.org/en/content/articlelanding/2019/ra/c9ra02140j]
- 59. Construction of an Acetylcholinesterase Sensor Based on ... [https://www.mdpi.com/1424-8220/17/4/676]
- 60. Covalent immobilization of Drosophila ... [https://www.ovid.com/journals/biab/pdf/10.1042/ba20080005~covalent-immobilization-ofdrosophilaacetylcholinesterase-for]
- 61. Flow-Through Acetylcholinesterase Sensor with ... [https://www.mdpi.com/2079-6374/12/9/676]
- 62. A Sensitive Electrochemical Cholinesterase-Inhibiting ... [https://pubmed.ncbi.nlm.nih.gov/41002315/]
- 63. A sensitive acetylcholinesterase biosensor based on ... [https://www.sciencedirect.com/science/article/abs/pii/S1572665721003556]
- 64. Pd-modified MXene enabled electrochemical sensing ... [https://www.sciencedirect.com/science/article/abs/pii/S0003267025010190]
- 65. An acetylcholinesterase-based biosensor of carbofuran ... [https://journal-iasssf.com/index.php/EAM/article/view/1963]
- 66. Acetylcholinesterase-immobilized cellulose membrane ... [https://www.sciencedirect.com/science/article/abs/pii/S1386142525003117]
- 67. Sensors | Free Full-Text | Immobilization of Acetylcholinesterase on Screen-Printed Electrodes. Application to the Determination of Arsenic(III) | HTML [https://www.mdpi.com/1424-8220/10/3/2119/htm]
- 68. An Amperometric Acetylcholine Biosensor Based on Co-Immobilization of Enzyme Nanoparticles onto Nanocomposite - PMC [https://pmc.ncbi.nlm.nih.gov/articles/PMC10046218/]
- 69. Development of a biosensor based on immobilization of acetylcholinesterase on NiO nanoparticles-carboxylic graphene-nafion modified electrode for detection of pesticides - PubMed [https://pubmed.ncbi.nlm.nih.gov/23708635/]
- 70. and characterization, and... Acetylcholinesterase immobilization [https://pubmed.ncbi.nlm.nih.gov/26839417/]
- 71. Optimization and evaluation of acetylcholine esterase ... [https://www.sciencedirect.com/science/article/abs/pii/S1359511309002566]
- 72. BVT SPE with immobilized Acetylcholinesterase [https://www.palmsens.com/product/ac1-ache/]
- 73. A novel RP-HPLC method for quantification of ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9803206/]
- 74. Measurement of acetylcholinesterase activity by ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC12417993/]
- 75. Micro-Electrometric Method for Assessing Cholinesterase ... [https://www.mdpi.com/2409-9279/8/2/30]
- 76. Screening of Acetylcholinesterase Inhibitors by Capillary ... [https://www.mdpi.com/1420-3049/29/1/118]
- 77. A new approach to develop a standardized method for ... [https://www.sciencedirect.com/science/article/abs/pii/S1570023214000956]
- 78. Online acetylcholinesterase inhibition evaluation by high-performance liquid chromatography–mass spectrometry hyphenated with an immobilized enzyme reactor - ScienceDirect [https://www.sciencedirect.com/science/article/abs/pii/S0021967319308908]
- 79. In vitro inhibition of acetylcholinesterase activity by yellow ... [https://pmc.ncbi.nlm.nih.gov/articles/PMC9635817/]
- 80. Influence of acetylcholinesterase immobilization on the ... [https://www.sciencedirect.com/science/article/abs/pii/S0169433214008964]
- 81. Acetylcholinesterase electrochemical biosensors with ... [https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0231981]
- 82. Portable, Energy-Autonomous Electrochemical Impedance ... [https://www.mdpi.com/2673-4591/87/1/89]